Principles of Biochemistry/Signaling inside the Cell
Cell signaling is part of a complex system of communication that governs basic cellular activities and coordinates cell actions. The ability of cells to perceive and correctly respond to their microenvironment is the basis of development, tissue repair, and immunity as well as normal tissue homeostasis. Errors in cellular information processing are responsible for diseases such as cancer, autoimmunity, and diabetes. By understanding cell signaling, diseases may be treated effectively and, theoretically, artificial tissues may be created. Traditional work in biology has focused on studying individual parts of cell signaling pathways. Systems biology research helps us to understand the underlying structure of cell signaling networks and how changes in these networks may affect the transmission and flow of information. Such networks are complex systems in their organization and may exhibit a number of emergent properties including bistability and ultrasensitivity. Analysis of cell signaling networks requires a combination of experimental and theoretical approaches including the development and analysis of simulations and modelling[1].
Signaling pathways inside the Cell
[edit | edit source]
In some cases, receptor activation caused by ligand binding to a receptor is directly coupled to the cell's response to the ligand. For example, the neurotransmitter GABA can activate a cell surface receptor that is part of an ion channel. GABA binding to a GABA A receptor on a neuron opens a chloride-selective ion channel that is part of the receptor. GABA A receptor activation allows negatively-charged chloride ions to move into the neuron, which inhibits the ability of the neuron to produce action potentials. However, for many cell surface receptors, ligand-receptor interactions are not directly linked to the cell's response. The activated receptor must first interact with other proteins inside the cell before the ultimate physiological effect of the ligand on the cell's behavior is produced. Often, the behavior of a chain of several interacting cell proteins is altered following receptor activation. The entire set of cell changes induced by receptor activation is called a signal transduction mechanism or pathway.[citation needed] In the case of Notch-mediated signaling, the signal transduction mechanism can be relatively simple. As shown in Figure 2 (above, left), activation of Notch can cause the Notch protein to be altered by a protease. Part of the Notch protein is released from the cell surface membrane and can act to change the pattern of gene transcription in the cell nucleus. This causes the responding cell to make different proteins, resulting in an altered pattern of cell behavior. Cell signaling research involves studying the spatial and temporal dynamics of both receptors and the components of signaling pathways that are activated by receptors in various cell types.[citation needed] A more complex signal transduction pathway is shown in Figure 3. This pathway involves changes of protein-protein interactions inside the cell, induced by an external signal. Many growth factors bind to receptors at the cell surface and stimulate cells to progress through the cell cycle and divide. Several of these receptors are kinases that start to phosphorylate themselves and other proteins when binding to a ligand. This phosphorylation can generate a binding site for a different protein and thus induce protein-protein interaction. In Figure 3, the ligand (called epidermal growth factor (EGF)) binds to the receptor (called EGFR). This activates the receptor to phosphorylate itself. The phosphorylated receptor binds to an adaptor protein (GRB2), which couples the signal to further downstream signaling processes. For example, one of the signal transduction pathways that are activated is called the mitogen-activated protein kinase (MAPK) pathway. The signal transduction component labeled as "MAPK" in the pathway was originally called "ERK," so the pathway is called the MAPK/ERK pathway. The MAPK protein is an enzyme, a protein kinase that can attach phosphate to target proteins such as the transcription factor MYC and, thus, alter gene transcription and, ultimately, cell cycle progression. Many cellular proteins are activated downstream of the growth factor receptors (such as EGFR) that initiate this signal transduction pathway.[citation needed] Some signaling transduction pathways respond differently depending on the amount of signaling received by the cell. For instance, the hedgehog protein activates different genes, depending on the amount of hedgehog protein present.[citation needed] Complex multi-component signal transduction pathways provide opportunities for feedback, signal amplification, and interactions inside one cell between multiple signals and signaling pathways[2].
Intercellular communication
[edit | edit source]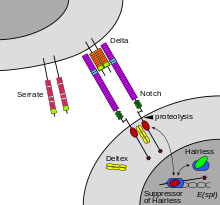
Intracrine refers to a hormone that acts inside a cell. Steroid hormones act through intracellular (mostly nuclear) receptors and, thus, are considered to be intracrines. In contrast, peptide or protein hormones, in general, act as endocrines, autocrines, or paracrines by binding to their receptors present on the cell surface. Several peptide/protein hormones or their isoforms also act inside the cell through different mechanisms. These peptide/protein hormones, which have intracellular functions, are also called intracrines. The term 'intracrine' is thought to have been coined to represent peptide/protein hormones that also have intracellular actions. The biological effects produced by intracellular actions are referred as intracrine effects, whereas those produced by binding to cell surface receptors are called endocrine, autocrine, or paracrine effects, depending on the origin of the hormone. The intracrine effect of some of the peptide/protein hormones are similar to their endocrine, autocrine, or paracrine effects; however, these effects are different for some other hormones[3].
Autocrine signaling is a form of signaling in which a cell secretes a hormone or chemical messenger (called the autocrine agent) that binds to autocrine receptors on the same cell, leading to changes in the cell. This can be contrasted with paracrine signaling, intracrine signaling, or classical endocrine signaling.An example of an autocrine agent is the cytokine interleukin-1 in monocytes. When interleukin-1 is produced in response to external stimuli, it can bind to cell-surface receptors on the same cell that produced it. Another example occurs in activated T cell lymphocytes, i.e., when a T cell is induced to mature by binding to a peptide:MHC complex on a professional antigen-presenting cell and by the B7:CD28 costimulatory signal. Upon activation, "low-affinity" IL-2 receptors are replaced by "high-affinity" IL-2 receptors consisting of α, β, and γ chains. The cell then releases IL-2, which binds to its own new IL-2 receptors, causing self-stimulation and ultimately a monoclonal population of T cells. These T cells can then go on to perform effector functions such as macrophage activation, B cell activation, and cell-mediated cytoxicity.
Juxtacrine signaling is a type of intercellular communication that is transmitted via oligosaccharide, lipid, or protein components of a cell membrane, and may affect either the emitting cell or the immediately-adjacent cells. It occurs between adjacent cells that possess broad patches of closely-opposed plasma membrane linked by transmembrane channels known as connexons. The gap between the cells can usually be between only 2 and 4 nm. Unlike other types of cell signaling (such as paracrine and endocrine), juxtacrine signaling requires physical contact between the two cells involved. Juxtacrine signaling has been observed for some growth factors, cytokine and chemokine cellular signals.
Endocrine signals target distant cells. Endocrine cells produce hormones that travel through the blood to reach all parts of the body.

Paracrine signaling is a form of cell signaling in which the target cell is near ("para" = near) the signal-releasing cell.Some signaling molecules degrade very quickly, limiting the scope of their effectiveness to the immediate surroundings. Others affect only nearby cells because they are taken up quickly, leaving few to travel further, or because their movement is hindered by the extracellular-matrix.Growth factor and clotting factors are paracrine signaling agents. The local action of growth factor signaling plays an especially important role in the development of tissues. Also, retinoic acid, the active form of vitamin A, functions in a paracrine fashion to regulate gene expression during embryonic development in higher animals. In insects, Allatostatin controls growth though paracrine action on the corpora allata. In mature organisms, paracrine signaling is involved in responses to allergens, tissue repair, the formation of scar tissue, and blood clotting.
MAPK/ERK pathway
[edit | edit source]The MAPK/ERK pathway is a chain of proteins in the cell that communicates a signal from a receptor on the surface of the cell to the DNA in the nucleus of the cell. The signal starts when a growth factor binds to the receptor on the cell surface and ends when the DNA in the nucleus expresses a protein and produces some change in the cell, such as cell division. The pathway includes many proteins, including MAPK (originally called ERK), which communicate by adding phosphate groups to a neighboring protein, which acts as an "on" or "off" switch. When one of the proteins in the pathway is mutated, it can be stuck in the "on" or "off" position, which is a necessary step in the development of many cancers. Components of the MAPK/ERK pathway were discovered when they were found in cancer cells. Drugs that reverse the "on" or "off" switch are being investigated as cancer treatments. Receptor-linked tyrosine kinases such as the epidermal growth factor receptor (EGFR) are activated by extracellular ligands. Binding of epidermal growth factor (EGF) to the EGFR activates the tyrosine kinase activity of the cytoplasmic domain of the receptor. The EGFR becomes phosphorylated on tyrosine residues. Docking proteins such as GRB2 contains an SH2 domain that binds to the phosphotyrosine residues of the activated receptor . GRB2 binds to the guanine nucleotide exchange factor SOS by way of the two SH3 domains of GRB2. When the GRB2-SOS complex docks to phosphorylated EGFR, SOS becomes activated. Activated SOS then promotes the removal of GDP from a member of the Ras subfamily (most notably H-Ras or K-Ras). Ras can then bind GTP and become active. Apart from EGFR, other cell surface receptors that can activate this pathway via GRB2 include Trk A/B, Fibroblast growth factor receptor (FGFR) and PDGFR[4].
Activated Ras activates the protein kinase activity of RAF kinase . RAF kinase phosphorylates and activates MEK. MEK phosphorylates and activates a mitogen-activated protein kinase (MAPK). RAF, MEK, and MAPK are all serine/threonine-selective protein kinases. In the technical sense, RAF, MEK, and MAPK are all mitogen-activated kinases, as is MNK (see below). MAPK was originally called "extracellular signal-regulated kinases" (ERKs) and "microtubule-associated protein kinase" (MAPK). One of the first proteins known to be phosphorylated by ERK was a microtubule-associated protein (MAP). As discussed below, many additional targets for phosphorylation by MAPK were later found, and the protein was re-named "mitogen-activated protein kinase" (MAPK). The series of kinases from RAF to MEK to MAPK is an example of a protein kinase cascade. Such series of kinases provide opportunities for feedback regulation and signal amplification.
Three of the many proteins that are phosphorylated by MAPK are shown in the Figure. One effect of MAPK activation is to alter the translation of mRNA to proteins. MAPK phosphorylates 40S ribosomal protein S6 kinase (RSK). This activates RSK, which, in turn, phosphorylates ribosomal protein S6 . Mitogen-activated protein kinases that phosphorylate ribosomal protein S6 were the first to be isolated. MAPK regulates the activities of several transcription factors. MAPK can phosphorylate C-myc. MAPK phosphorylates and activates MNK, which, in turn, phosphorylates CREB. MAPK also regulates the transcription of the C-Fos gene. By altering the levels and activities of transcription factors, MAPK leads to altered transcription of genes that are important for the cell cycle. The 22q11, 1q42, and 19p13 genes are associated with schizophrenia, schizoaffective, bipolar, and migraines by affecting the ERK pathway.
In simpler terms, the mitogen binds to the membrane ligand. This means that Ras (a GTPase) can swap its GDP for a GTP. It can now activate MAP3K (e.g., Raf), which activates MAP2K, which activates MAPK. MAPK can now activate a transcription factor, such as myc[5].
What is Extracellular-signal-regulated kinases (ERKs)?
[edit | edit source]In biochemistry, extracellular-signal-regulated kinases (ERKs)(old name of MAPK) or classical MAP kinases are widely expressed protein kinase intracellular signalling molecules that are involved in functions including the regulation of meiosis, mitosis, and postmitotic functions in differentiated cells. Many different stimuli, including growth factors, cytokines, virus infection, ligands for heterotrimeric G protein-coupled receptors, transforming agents, and carcinogens, activate the ERK pathway. The term, "extracellular-signal-regulated kinases", is sometimes used as a synonym for mitogen-activated protein kinase (MAPK), but has more recently been adopted for a specific subset of the mammalian MAPK family. In the MAPK/ERK pathway, Ras activates c-Raf, followed by mitogen-activated protein kinase kinase (abbreviated as MKK, MEK, or MAP2K) and then MAPK1/2 (below). Ras is typically activated by growth hormones through receptor tyrosine kinases and GRB2/SOS, but may also receive other signals. ERKs are known to activate many transcription factors, such as ELK1, and some downstream protein kinases. Disruption of the ERK pathway is common in cancers, especially Ras, c-Raf and receptors such as HER2.
Mitogen-activated protein kinase 1 (MAPK1) is also known as "extracellular signal-regulated kinase 2" (ERK2). Two similar (85% sequence identity) protein kinases were originally called ERK1 and ERK2. They were found during a search for protein kinases that are rapidly phosphorylated after activation of cell surface tyrosine kinases such as the epidermal growth factor receptor. Phosphorylation of ERKs leads to the activation of their kinase activity. The molecular events linking cell surface receptors to activation of ERKs are complex. It was found that Ras GTP-binding proteins are involved in the activation of ERKs. Another protein kinase, Raf-1, was shown to phosphorylate a "MAPK kinase", thus qualifying as a "MAPK kinase kinase". The MAPK kinase was named "MAPK/ERK kinase" (MEK). Receptor-linked tyrosine kinases, Ras, Raf, MEK, and MAPK could be fitted into a signaling cascade linking an extracellular signal to MAPK activation. See: MAPK/ERK pathway. Transgenic gene knockout mice lacking MAPK1 have major defects in early development.
Mitogen-activated protein kinase 3 (MAPK3) is also known as "extracellular signal-regulated kinase 1" (ERK1). Transgenic gene knockout mice lacking MAPK3 are viable and it is thought that MAPK1 can fulfill most MAPK3 functions in most cells. The main exception is in T cells. Mice lacking MAPK3 have reduced T cell development past the CD4+CD8+ stage[6].
Activation of Mitogen-activated protein (MAP) kinases
[edit | edit source]MAP kinases are activated within the protein kinase cascades called “MAPK cascade”. Each one consists of three enzymes, MAP kinase, MAP kinase kinase (MKK, MEK, or MAP2K) and MAP kinase kinase kinase (MKKK, MEKK or MAP3K) that are activated in series. A MAP3K that is activated by extracellular stimuli phosphorylates a MAP2K on its serine and threonine residues, and this MAP2K activates a MAP kinase through phosphorylation on its threonine and tyrosine residues (Tyr-185 and Thr-183 of ERK2). In vivo and in vitro, phosphorylation of tyrosine precedes phosphorylation of threonine, although phosphorylation of either residue can occur in the absence of the other. Because both tyrosine and threonine phosphorylations are required to activate the MAP kinases, phosphatases that remove phosphate from either site will inactivate them.
The MAP kinase signaling cascade has been well-conserved in evolution from yeast to mammals. Cascades convey information to effectors, coordinate incoming information from other signaling pathways, amplify signals, and allow for a variety of response patterns. They respond to different stimuli by phosphorylating cytoplasmic components and nuclear transcription factors depending on the cellular context. Down-regulation of MAP kinase pathways may occur through dephosphorylation by serine/threonine phosphatases, tyrosine phosphatases, or dual-specificity phosphatases and through feedback inhibitory mechanisms that involve the phosphorylation of upstream kinases. Drugs that selectively down-regulate MAP kinase cascades could prove to be valuable as therapeutic agents in the control of malignant disease[7].
Groups
[edit | edit source]ERK1 and ERK2 were the first of the ERK/MAP kinase subfamily to be cloned. Other related mammalian enzymes have been detected including: two ERK3 isoforms, ERK4, Jun N-terminal kinases/stress-activated protein kinases (JNK/SAPKs), p38/HOG, and p57 MAP kinases (38). The presence of at least six MAP kinases in yeast suggests that there are more in mammals.
- extracellular signal-regulated kinases (ERK1, ERK2). The ERK1/2 (also known as classical MAP kinases) signaling pathway is preferentially activated in response to growth factors and phorbol ester (a tumor promoter), and regulates cell proliferation and cell differentiation.
- c-Jun N-terminal kinases (JNKs), (MAPK8, MAPK9, MAPK10) also known as stress-activated protein kinases (SAPKs).
- p38 isoforms.(p38-α (MAPK14), -β (MAPK11), -γ (MAPK12 or ERK6) and -δ (MAPK13 or SAPK4)) Both JNK and p38 signaling pathways are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock, and are involved in cell differentiation and apoptosis.
- ERK5. ERK5 (MAPK7), which has been found recently, is activated both by growth factors and by stress stimuli, and it participates in cell proliferation.
- ERK3/4. ERK3 (MAPK6) and ERK4 (MAPK4) are structurally-related atypical MAPKs possessing SEG motifs in the activation loop and displaying major differences only in the C-terminal extension. ERK3 and ERK4 are primarily cytoplasmic proteins that bind, translocate, and activate MK5 (PRAK, MAPKAP5). ERK3 is unstable, unlike ERK4, which is relatively stable.[8]
- ERK7/8. (MAPK15) This is the newest member of MAPKs and behaves like atypical MAPKs. It possesses a long C terminus similar to ERK3/4[9].
c-Jun N-terminal kinases (JNKs)
[edit | edit source]c-Jun N-terminal kinases (JNKs), were originally identified as kinases that bind and phosphorylate c-Jun on Ser-63 and Ser-73 within its transcriptional activation domain. They belong to the mitogen-activated protein kinase family, and are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock. They also play a role in T cell differentiation and the cellular apoptosis pathway. Activation occurs through a dual phosphorylation of threonine (Thr) and tyrosine (Tyr) residues within a Thr-Pro-Tyr motif located in kinase subdomain VIII. Activation is carried out by two MAP kinases, MKK4 and MKK7 and JNK can be inactivated by Ser/Thr and Tyr protein phosphatases. It has been suggested that this signaling pathway contributes to inflammatory responses in mammals and insects. Inflammatory signals, changes in levels of reactive oxygen species, ultraviolet radiation, protein synthesis inhibitors, and a variety of stress stimuli can activate JNK. One way this activation may occur is through disruption of the conformation of sensitive protein phosphatase enzymes; specific phosphatases normally inhibit the activity of JNK itself and the activity of proteins linked to JNK activation. JNKs can associate with scaffold proteins JNK interacting proteins as well as their upstream kinases JNKK1 and JNKK2 following their activation. JNK, by phosphorylation, modifies the activity of numerous proteins that reside at the mitochondria or act in the nucleus. Downstream molecules that are activated by JNK include c-Jun, ATF2, ELK1, SMAD4, p53 and HSF1. The downstream molecules that are inhibited by JNK activation include NFAT4, NFATC1 and STAT3. By activating and inhibiting other small molecules in this way, JNK activity regulates several important cellular functions including cell growth, differentiation, survival and apoptosis. JNK1 is involved in apoptosis, neurodegeneration, cell differentiation and proliferation, inflammatory conditions and cytokine production mediated by AP-1 (activation protein 1) such as RANTES, IL-8 and GM-CSF. Recently, JNK1 has been found to regulate Jun protein turnover by phosphorylation and activation of the ubiquitin ligase Itch[10].
Control of the MAP kinase cascade
[edit | edit source]Receptor tyrosine kinase
[edit | edit source]Various ligands that activate MAPK’s cascade bind receptor tyrosine kinases, and tyrosine residues are phosphorylated; the phosphotyrosine residues of autophosphorylated receptors then bind the SH2 domains of adapters, (Grb2: growth factor receptor-bound protein 2). Exchange factors promote the association of Ras with GTP. GTP-Ras bind Raf-1 and B-Raf, two protein kinases. Consequently, Raf protein kinase activity is increased. Receptor tyrosine kinases have also been reported to activate the cascade in fibroblasts via a [Ca2+] increase.
G Protein-coupled receptors
[edit | edit source]The MAP kinase cascade can also be activated by certain heterotrimeric G proteins.
Protein kinase C
[edit | edit source]Protein kinase C (PKC) is used by many receptors to regulate the MAP kinase pathway, alone or in concert with other mechanisms, and may act at several steps in the cascade. PKC may directly activate Raf-1, but if a mutation exists at the site phosphorylated by PKC, no interaction can occur with Raf. Other sites of action of PKC are likely to be either farther upstream or at the level of MAP kinase inactivation.
Regulation and specificity of MEKs
[edit | edit source]MEK1 and MEK2 phosphorylate and activate MAP kinase. MEKs are activated by Raf-1, B-Raf, the Mos protooncogene product, MEK kinase 1 (MEKK1), and other growth factor-stimulated activities. The mechanisms controlling MEKK1 are unknown, although Ras may be required. It is thought MEKs are kinases that phosphorylate only MAP Kinases because no other substrates have been identified.
JAK-STAT signaling pathway
[edit | edit source]
The JAK-STAT signaling pathway transmits information from chemical signals outside the cell, through the cell membrane, and into gene promoters on the DNA in the cell nucleus, which causes DNA transcription and activity in the cell. The JAK-STAT system is a major signaling alternative to the second messenger system. The JAK-STAT system consists of three main components: a receptor, JAK and STAT.[11]
JAK is short for Janus Kinase, and STAT is short for Signal Transducer and Activator of Transcription.[11]
The receptor is activated by a signal from interferon, interleukin, growth factors, or other chemical messengers. This activates the kinase function of JAK, which autophosphorylates itself (phosphate groups act as "on" and "off" switches on proteins). The STAT protein then binds to the phosphorylated receptor. STAT is phosphorylated and translocates into the cell nucleus, where it binds to DNA and promotes transcription of genes responsive to STAT.
In mammals, there are seven STAT genes, and each one binds to a different DNA sequence. STAT binds to a DNA sequence called a promoter, which controls the expression of other DNA sequences. This affects basic cell functions, like cell growth, differentiation and death.[11][12]
The JAK-STAT pathway is evolutionarily conserved, from slime molds and worms to mammals (but not fungi or plants). Disrupted or dysregulated JAK-STAT functionality (which is usually by inherited or acquired genetic defects) can result in immune deficiency syndromes and cancers.[11]
Mechanism
[edit | edit source]JAKs, which have tyrosine kinase activity, bind to some cell surface cytokine receptors. The binding of the ligand to the receptor triggers activation of JAKs. With increased kinase activity, they phosphorylate tyrosine residues on the receptor and create sites for interaction with proteins that contain phosphotyrosine-binding SH2 domains. STATs possessing SH2 domains capable of binding these phosphotyrosine residues are recruited to the receptors, and are themselves tyrosine-phosphorylated by JAKs. These phosphotyrosines then act as binding sites for SH2 domains of other STATs, mediating their dimerization. Different STATs form hetero- or homodimers. Activated STAT dimers accumulate in the cell nucleus and activate transcription of their target genes.[13] STATs may also be tyrosine-phosphorylated directly by receptor tyrosine kinases, such as the epidermal growth factor receptor, as well as by non-receptor tyrosine kinases such as c-src.
The pathway is negatively regulated on multiple levels. Protein tyrosine phosphatases remove phosphates from cytokine receptors and activated STATs.[13] More recently identified suppressors of cytokine signalling (SOCS) inhibit STAT phosphorylation by binding and inhibiting JAKs or competing with STATs for phosphotyrosine binding sites on cytokine receptors.[14] STATs are also negatively regulated by protein inhibitors of activated STAT (PIAS), which act in the nucleus through several mechanisms.[15] For example, PIAS1 and PIAS3 inhibit transcriptional activation by STAT1 and STAT3 respectively by binding and blocking access to the DNA sequences they recognize[16].
Janus kinase inhibitors
Janus kinase inhibitor is a class of medicines that function by inhibiting the effect of one or more of the Janus kinase family of enzymes (JAK1, JAK2, JAK3, TYK2), interfering with the JAK-STAT signaling pathway.
Some JAK2 inhibitors are under development for the treatment of polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Some inhibitors of JAK2 are in clinical trials, e.g. for psoriasis.
JAK3 is also being targeted for a variety of inflammatory diseases, and one has had good results in a phase II trial for rheumatoid arthritis.
Examples
* Lestaurtinib against JAK2, for acute myelogenous leukemia (AML) * Tofacitinib (previously called tasocitinib) (CP-690550) against JAK3 for psoriasis, and rheumatoid arthritis.Early Phase III results in November 2010 were encouraging.[6] * Ruxolitinib[7] against JAK1/JAK2 for psoriasis, myelofibrosis, and rheumatoid arthritis * SB1518[11][12] against JAK2 for relapsed lymphoma, advanced myeloid malignancies, myelofibrosis and CIMF * CYT387 against JAK2 for myeloproliferative disorders * LY3009104 (INCB28050) against JAK1/JAK2 starting phase IIb for rheumatoid arthritis * TG101348 against JAK2; phase I results for myelofibrosis are published[
Refrences
[edit | edit source]- ↑ http://en.wikipedia.org/w/index.php?title=Cell_signaling&oldid=424060709
- ↑ http://en.wikipedia.org/w/index.php?title=Cell_signaling&oldid=424060709
- ↑ http://en.wikipedia.org/w/index.php?title=Intracrine&oldid=405759955
- ↑ http://en.wikipedia.org/w/index.php?title=MAPK/ERK_pathway&oldid=422032501
- ↑ http://en.wikipedia.org/w/index.php?title=MAPK/ERK_pathway&oldid=422032501
- ↑ http://en.wikipedia.org/w/index.php?title=Extracellular_signal-regulated_kinases&oldid=414776335
- ↑ http://en.wikipedia.org/w/index.php?title=Mitogen-activated_protein_kinase&oldid=421970562
- ↑ Kant S, Schumacher S, Singh MK, Kispert A, Kotlyarov A, Gaestel M. (2006). "Characterization of the atypical MAPK ERK4 and its activation of the MAPK-activated protein kinase MK5". J Biol Chem. 281 (46): 35511–9. doi:10.1074/jbc.M606693200. PMID 16973613.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ http://en.wikipedia.org/w/index.php?title=Mitogen-activated_protein_kinase&oldid=421970562
- ↑ http://en.wikipedia.org/w/index.php?title=C-Jun_N-terminal_kinases&oldid=417136418
- ↑ a b c d Aaronson DS, Horvath CM (2002). "A road map for those who don't know JAK-STAT". Science. 296 (5573): 1653–5. doi:10.1126/science.1071545. PMID 12040185.
{{cite journal}}: Unknown parameter|month=ignored (help) - ↑ http://en.wikipedia.org/w/index.php?title=JAK-STAT_signaling_pathway&oldid=416185051
- ↑ a b Hebenstreit D, Horejs-Hoeck J and Duschl A (2005). "JAK/STAT-dependent gene regulation by cytokines". Drug News Perspect. 18 (4): 243–249. doi:10.1358/dnp.2005.18.4.908658. PMID 16034480. 16034480.
- ↑ Krebs DL, Hilton DJ (2001). "SOCS proteins: negative regulators of cytokine signaling". Stem Cells. 19 (5): 378–87. doi:10.1634/stemcells.19-5-378. PMID 11553846.
- ↑ Shuai K (2006). "Regulation of cytokine signaling pathways by PIAS proteins". Cell Research. 16 (2): 196–202. doi:10.1038/sj.cr.7310027. PMID 16474434. 16474434.
- ↑ http://en.wikipedia.org/w/index.php?title=JAK-STAT_signaling_pathway&oldid=416185051