Planet Earth/5b. Properties of Earth’s Water (Density, Salinity, Oxygen, and Carbonic Acid)
Properties of Earth’s Water
[edit | edit source]As one of the most abundant molecules on Earth’s surface, and one vital to life, water plays an important role in the very dynamic nature of Earth, particularly how water interacts with the rocks, the soil, and the atmosphere, and how it changes depending on its depth in the Earth’s oceans.
Water Density
[edit | edit source]
Density is the mass per unit volume of a substance, which means that an equal volume of a substance with high density will weigh more than an equal volume of a substance with low density. Liquid water has a density of 1 gram per milliliter (1 g/ml) or 1 gram per cubic centimeter (1 g/cm3). This makes comparing the density of water to other substances, both liquids and solids easy. Specific density is the density of water compared to other substances. For example, a solid cube that floats in a glass of water, will have a specific density less than 1, while a solid cube that sinks will have a specific density of more than 1. A hydrometer is a special tool used to measure the specific density of water or other liquids in a solution by floating a standardized weight in the water to be measured. Liquids with different specific densities will organize in layers, with the least dense liquid at the top and most dense at the bottom. A mixture of liquids with different densities may take time to form layers as they reach equilibrium, and as long as the individual liquids do not go into solution with each other. In large bodies of water on Earth, differences in densities can stratify the water in lakes and oceans into different layers with depth.

Water’s density varies with temperature, such that pure water will have a density of 1 at 4°C, but water heated to the boiling point will have a density of 0.959 at 100°C. This means warm water will float on top of cold water. If the water is heated from the bottom, convection will cause the warm water to rise and more evenly distribute heat, however if water is heated at the surface, the warmer water will remain near the surface because it will be less dense than water at deeper colder layers. Water found in hot climates heated by the sun at the surface, will be more stratified (layered) than water heated by hydrothermal magma from below, which will cause the warm water to circulate convectively with surface waters and become mixed.
One strange aspect of water is that when it approaches the freezing temperature, the ultra-cold water near freezing and frozen water will be less dense, and float. It may seem like an obvious observation, that ice floats, especially if you have ever had a glass of ice water. But most substances when they freeze into a solid, will sink, as solids are typically denser then the liquid phase. Water is strange in that at about 4°C water is slightly denser than water at 0°C. In oceans and lakes and other large bodies of water as the water gets close to freezing it becomes slightly less dense and will rise from the bottom. Before it can freeze into a solid, the water will rise up to the surface and float. Frozen water, in the form of ice is only found near the surface as the top layer of bodies of water on Earth. This results in the floating ice on water to act as insulation to the warmer water below, due to the high heat capacity of the liquid water and high latent heat of the ice above. If frozen water sank, the bottom of oceans would be covered in thick layers of ice, and prevent life from living on the ocean floor.

Hydrogen bonding can explain why ice is less dense than liquid water. As water molecules become linked together in a crystal lattice structure as they freeze, they are packed less densely together with hydrogen atoms linked to oxygen atoms. The individual molecules become arranged in a hexagonal crystalline structure, with each angle of the hexagon represented by an oxygen atom. This crystalline structure is approximately 8.3% less dense than liquid water, and when frozen will expand in volume about 9% more than liquid water. This is why leaving a soda can in a freezer will result in an exploded open can, because the water when it freezes takes up more volume. The effect of the expansion of the freeze-thaw cycle of water is important in rock weathering on Earth’s surface especially in temperate climatic zones.
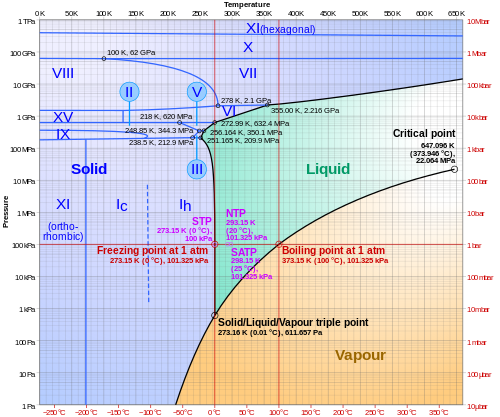
There are 18 known solid crystalline phases of ice, which can also form amorphous solid states, with inclusions of air and various gasses during its formation. Most ice on Earth’s surface is classified as ice-phase-1, or Ice Ih, at extremely cold temperatures and high pressures other types of crystalized ice will form. Most liquids under increasing pressures will freeze at higher temperatures because the pressure pushes the individual molecules tightly together. However, the hydrogen bonds in water resist this increased pressure, and results in a slight melting of ice at high pressure, below 0 °C. This peculiarity of ice will contribute to the motion of glaciers and ice sheets on Earth’s surface, by imposing a thin layer of liquid melt water which forms at the base of thick ice sheets.
Salinity
[edit | edit source]
Salinity is a measurement of the amount of salt dissolved in a body of water. Water with salt dissolved within it is called saline water. This is typically measured by the number of grams of salt that are found in 1,000 grams of water. Typical sea water has a salinity around 35 g/kg (sometimes written as parts per thousand, or 35 ppt). Drinking water has very low salinity of just 0 to 0.5 g/kg. The saltiest portions of the ocean include the Red Sea, where ocean salinity can get up to 40 g/kg, as well as in the Mediterranean Sea at 38 g/kg, which is due to the hot climates and enhanced evaporation over these portions of the ocean. The Great Salt Lake in Utah has salinity levels that range from 50 to 270 g/kg, making it significantly saltier than sea water.

The saltiest body of water on Earth is Don Juan Pond, a small lake found in a dry glacial valley near the McMurdo Station in Antarctica. Salinity in the lake is 400 g/kg (or 40% by weight). The extremely high salinity of Don Juan Pond allows the lake to be liquid, even in the freezing cold of Antarctica. The water is so enriched with salt that it has a viscosity of syrup. Viscosity is a measure of how well a liquid flows.

The addition of salt to water lowers the freezing point of water by a process called freezing point depression. The freezing point depression occurs because the more random the nature in a solution of water with various dissolved salts, the more opposed it will be to freezing, so that lower temperatures must be reached before the liquid will form a solid. The freezing point of water is lowered when the salt is added, as the salt makes it more difficult for water to freeze. This is why salt is often sprayed on roads and sidewalks to prevent ice in the winter.
Water with a salinity of 100 g/kg will freeze at -6°C, rather than 0°C. It is important to note that a solution containing water with salt as it freezes will not incorporate any of the salt within the crystal lattice, and instead the remaining water will become saltier over time. Just like boiling water where dissolved salts become enriched in the remaining water through distillation, freezing water can also be used to remove much of the salt content of the water, as the frozen water will contain no salts.

Dissolved salts in ocean water is not limited to sodium chloride (NaCl), table salt, but also includes sulfate (SO24-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+) ions. If you were to boil ocean water through distillation, the salts that would be left behind would be a mixture of table salt (NaCl), anhydrite (CaSO2), gypsum (CaSO4·2H2O), magnesium sulfate MgSO4·7H2O also known as Epsom salt, and potassium sulfate (K2SO4) which is also called potash. Many of these salts are mined from regions on Earth where oceans or large bodies of water evaporated leaving behind these salts deposited in rock layers now buried underground.

The higher the salinity of water, the greater its density. In the ocean, water at depth tends to be saltier than water found near the surface of the ocean, this is due, in part to the continuous flow of freshwater that enters the ocean from land and will float on its surface. The density of surface ocean water can increase underneath the formation of sea ice. As the ice forms on the surface of the ocean, the remaining cold ocean water just below the icy surface will become saltier, and will tend to sink due to the combined increase in salinity and cold temperature making the ocean water denser below sea ice.

Salinity of the Earth’s Oceans Over Time
[edit | edit source]
The ocean is salty because of the gradual concentration of dissolved ions that erode from the land and are washed into the sea. As such, scientists such as Edmond Halley in the early 1700s proposed that the oceans act as a net reservoir for the world’s salt, and that throughout Earth’s long history the oceans have increased in salinity over time. Little was known of variation in ocean salinity, but in 1872 the Royal Society converted a naval warship, the HMS Challenger to voyage around the world and study the ocean, collecting water samples, and dredging the ocean floor for deep sea life. The ship was equipped with oceanographic laboratories, state-of-art microscopes, and scientific tools to better understand the world’s oceans. From 1872 to 1876, the ship traveled around the world gathering samples of ocean water and recording its temperature. The samples were sent to a professor of chemistry, a German named William Dittmar for analysis. The results of the study demonstrate that the constitutes of the salts dissolved in the oceans do not vary greatly from region to region. Each sample had nearly equal amounts of Chloride (Cl) 19.350 ppt, Sodium (Na) 10.710 ppt, Sulfate (SO4) 2.690 ppt, Magnesium (Mg) 1.304 ppt, Calcium (Ca) 0.419 ppt, Potassium (K) 0.390 ppt, Bicarbonate (HCO3) 0.146 ppt, and Bromide (Br) 0.070 ppt, for a total of 35.079 ppt, or a salinity near 35 g/kg or ppt.

One of the surprising discoveries was when these values were compared to freshwater from rivers entering the ocean, Dittmar found significantly more Calcium (Ca) and Bicarbonate (HCO3), compared to Sodium (Na) and Chloride (Cl). So why does ocean water contain less of these two salts? The reason is that both Calcium and Bicarbonate are used by organisms to build their aragonite and calcite shells, using calcium carbonate (CaCO3). Over time these organisms die, the empty shells fall to the ocean floor and are buried, forming limestones. There is a cycle over long periods of time, in which limestones are uplifted onto the land, erode into Calcium (Ca) and Bicarbonate (HCO3), wash into the ocean, and are again used by organisms to build their shells, and then redeposited. This cycle likely has resulted in the oceans salinity over time to reach an equilibrium. Sodium (Na) and Chloride (Cl) are also removed from the oceans during burial, when inlets and shallow seas evaporate, leaving a salty crust that becomes buried. Although it is difficult to estimate salinity over all of Earth’s history, it is likely that salinity of the oceans during the Phanerozoic Eon (last 541 million years) has not changed much because of these processes.

Oxygen
[edit | edit source]Oxygen is necessary for aerobic animals, which require a source of oxygen for respiration, even underwater. Oxygen makes up 20.95% of the atmosphere. Oxygen, as a gas gets mixed into the ocean near its surface, where this dissolved oxygen gas is greater than about 7 parts per million (ppm). Ocean water with 10 ppm or greater is considered well-oxygenated water able to support a healthy population of fish. The amount of oxygen however decreases with depth, with deep ocean water becoming depleted in oxygen, from hypoxic (low oxygen) to anoxic (no oxygen) bottom waters. This results in fewer aerobic animals living in these deep ocean waters, which are dominated by anaerobic organisms, those are organisms that do not require the presence of oxygen to live.
The amount of oxygen dissolved in the ocean is also related to the water’s salinity and temperature. As salinity increases, the amount of gas dissolved decreases because more water molecules are linked up with calcium and sodium ions. As water temperature increases, the heat increases the mobility of oxygen dissolved gas molecules allowing them to more easily escape from the water, reducing the amount of oxygen. This is why healthy fish populations are never found in salty and extremely warm waters. The colder and fresher the ocean water, the more availability there is for oxygen gas to be present. This is why estuaries, where cold freshwater flows into the oceans are such healthy regions for large schools of fish and other aerobic life forms in the oceans.


Oxygen solubility is a function of pressure and temperature, and at sea level (with 1 atmosphere of pressure), the amount of oxygen found within the ocean surface water is directly related to the temperature of the ocean water at its surface. Pure water near freezing temperatures (0° C) can hold upwards of 14 ppm oxygen, at 25° C (77° F), water holds about 8 ppm oxygen, however, when warm water is heated to 45° C (113° F) it only holds 6 ppm oxygen. As water approaches the boiling point 100° C (212° F) little to no oxygen can be dissolved in the water. If you have ever boiled water, you may have noticed that as the water is heated, tiny air bubbles start to form, because the warmed water cannot hold the dissolved oxygen gas. At 100° C (212° F) the water itself starts to boil, and the large bubbles are formed by evaporation of water. However, if the water is cooled back down and then again reheated, bubbles will not appear until the water starts to boil, because the oxygen and other gasses have already escaped from the water during the first time the water was heated.
Since ocean water has a higher salinity than pure water, it is more susceptible to the loss of oxygen. With a salinity of 35 g/kg or ppt, most ocean water will fall below 10 ppm oxygen, and in areas of high salinity where there is increased evaporation and little wave action to contribute oxygen, ocean water near the surface can become deoxygenated. Deoxygenated surface waters are referred to as “dead zones,” because fish lack the necessary oxygen for respiration and die on mass. Dead zones can manifest when lakes or seas become too hot and salty, and lack mixing from crashing waves. In 2017, a dead zone in the Gulf of Mexico was observed when oxygen levels fell below 2 ppm and extended over 8,776 square miles of the coast of Texas, killing fish and shrimp that need more oxygen dissolved in the water. Fear exists that with rising global temperatures, dead zones, such as this one will become more common, and could destroy the fishing industry.
Another important factor in the amount of oxygen available in water is eutrophication. Eutrophication is when a body of water becomes enriched in fertilizers (nitrogen, phosphorus, and sulfate) which results in excess growth of algae on a water’s surface. Algae, which photosynthesizes and produces oxygen. Knowing this fact, it would seem that a healthy coat of algae on the surface of water would increase the amount of oxygen in the water. However, the excessive algae growth on the surface blocks light from reaching deeper depths in the water, resulting in a zone just below the algae covered surface where photosynthesizing lifeforms cannot grow. This dark zone becomes depleted in oxygen over time, and accumulates layers of dead algae that sinks into these dark waters. This graveyard of decaying algae supports scavenging microbial bacteria that feed on this decaying organic matter and thrive in the anoxic deep waters. Eutrophication is a major problem for bodies of water that receive run off from agricultural land where fertilizers have been used heavily.

Carbon dioxide
[edit | edit source]
Carbon dioxide is necessary for photosynthesizing lifeforms, such as cyanobacteria, algae, and phytoplankton, which require both sunlight and a source of carbon dioxide for respiration and growth. However, carbon dioxide is much rarer in the atmosphere (250 to 410 ppm), but quickly rising over the last century. Just like oxygen, carbon dioxide as a gas enters the ocean by agitations of the surface by wind and crashing waves. However, one important aspect of carbon dioxide, that differs from oxygen gas is that it also reacts with water to form carbonic acid, by the chemical equation CO2 + H2O → H2CO3 (carbonic acid). This reaction can go both ways, and release carbon dioxide. Although the amount of carbon dioxide that undergoes this reaction is rather small. In water with carbon dioxide at 25° C (77° F), the ratio of carbonic acid to carbon dioxide, is just 0.0017, and in ocean water 0.0012, which means that most of the carbon dioxide is dissolved as a gas. In the upper parts of the ocean, carbon dioxide ranges greatly between 150 to 700 μatm partial pressure (pCO2), with typical numbers between 280 to 320 pCO2, but has been increasing by about 1.3 ± 0.5 μatm per year on average [Takahashi et al. 1997], in line with increases in atmospheric carbon dioxide over the last century.

Just like oxygen gas, carbon dioxide solubility is a function of pressure and temperature, and at sea level (with 1 atmosphere of pressure), the amount of carbon dioxide found within the ocean surface water is directly related to the temperature of the ocean water at its surface. The warmer the ocean water becomes the less carbon dioxide gas the water will be able to hold. There is a fear that with increasing carbon dioxide in the atmosphere and warming global temperatures, the ocean will no longer act like a sink or reservoir for carbon dioxide gas, and the oceans will reach a saturation point with respect to carbon dioxide. If this were to happen, the amount of carbon dioxide in the atmosphere would rise more dramatically, since less carbon dioxide would able to be dissolved in the oceans. However, carbon dioxide reacts with water to form H2CO3 (carbonic acid), which would result in the continued capacity for the absorption of carbon dioxide. The product of this reaction, carbonic acid, will release more hydrogen H+ ions (H2CO3 -> H+ + HCO3- ), resulting in the ocean water becoming more acidic over time. Ocean water is slightly basic, with a pH of 8.12, which has been dropping in recent years to a pH of 8.06 since the 1990s. The slightly basic nature of ocean water is largely due to the excess bicarbonate (HCO3- ) ions that erode from land and are washed into the oceans by rivers. Normally with pH greater than 8, the excess bicarbonate (HCO3- ) ions further contribute a hydrogen ion (H+) resulting in carbonate ions (CO3-2), when this occurs the carbonate ions react to any Ca+2 forming CaCO3 which precipitates out as a solid. You may have noticed this type of reaction if you have used a household cleaner to clean a bathtub. Most cleaners contain calcium carbonate and calcium hydroxide, the calcium hydroxide keeps the pH very high, resulting in any excess of calcium Ca+2 ions in the water to precipitate out as a solid, as white flakes of calcium carbonate CaCO3 you might observe in the rinse water after cleaning. However, if the pH becomes less than 8, the bicarbonate (HCO3- ) ions no longer contribute an extra hydrogen ion (H+), and carbonate ions (CO3-2) no longer form. This results in a lack of deposition of calcium carbonate, and most of the bicarbonate (HCO3- ) ions would remain in solution in ocean water with pH lower than 8.
Oceanographers worry that as the Earth’s oceans become more enriched in H2CO3 (carbonic acid) and become increasingly acidic, that its carbonate chemistry is going to fundamentally change. With ocean depth, water increases in pressure and decreases in temperature, resulting in the dissolution of CaCO3. The carbonate compensation depth (CCD) is defined as the water depth at which the rate of supply of CaCO3 from the surface is equal to the rate of dissolution into Ca+ and CO3-2 ions. Below this depth, CaCO3 dissolves in the water. Organisms that live below the CCD depth in the ocean are unable to compose their shells with calcite or aragonite the two minerals that are made of CaCO3, some deep-water sponges use silica instead, but most reef building organisms cannot live in water that is below the CCD depth.
The geological record contains several examples of deep-sea calcium carbonate (CaCO3) dissolution events that were driving by acidification of the oceans, for example, the Paleocene–Eocene Thermal Maximum (55.5 million years ago), and the Permian-Triassic boundary (252 million years ago). During these global warming events, where the atmosphere was enriched in carbon dioxide, calcium carbonate deposition was dramatically reduced. This can be observed in ancient ocean sediments bracketing these events, where calcium carbon rich limestones are replaced by silica and clay rich mudstone and claystone. Tropical coral reefs are heavily impacted by such events, as these communities require ocean water that can support healthy amounts of calcium carbonate used to make calcite and aragonite skeletons for coral reef communities. When carbon dioxide increases in the world’s oceans, the coral reefs in tropical environments vanish, and most colonial organisms that live in these marine environments become extinct.
| Previous | Current | Next |
|---|---|---|
|
b. Properties of Earth’s Water (Density, Salinity, Oxygen, and Carbonic Acid). |