Planet Earth/4e. Blaise Pascal and his Barometer

In 1647, Blaise Pascal living in Paris, France, was a celebrated genius in mathematics and inventor of the first mechanical calculator, when he took an interest in a great scientific debate. Some scientists argued that a complete vacuum was impossible. These scientists implied that it was impossible to remove all evidence of substances, including gas from a sealed glass container. There would be some substance left inside, no matter how hard you sucked out the gas from the glass container. Blaise Pascal argued that this idea was wrong. A vacuum was possible, and so he wrote a small pamphlet on the subject entitled Expériences nouvelles touchant le vide, or New Experiments with the Vacuum. Throughout the book Pascal described experiments with pipes, syringes, bellows and siphons, with a variety of liquids, including quicksilver (mercury). Pascal developed the modern scientific idea of physical forces exerting against substances, called pressure, which today is measured in the units of pascal. One pascal is equivalent to one newton of force applied over an area of one square meter.

One particular experiment excited Blaise Pascal more than any other: A series of experiments had been conducted in Italy in the 1630s and 1640s that showed how water would stay inside a sealed glass tube, even when it was opened from the bottom and allowed to drain into a basin. This failure to drain all the water into the basin suggested that some pressure was holding the water up inside the tube. Evangelista Torricelli, a student of Galileo, suggested that air had weight, and was pushing down against the liquid in the basin, which prevented the water from flowing out of the glass tube. Torricelli began experimenting with a new device filled with mercury, rather than water. Mercury worked better, because experiments with water required very long tubes (over 10 meters) and big water basins to work. Mercury worked well since the length of the tube could be short, enough to move the device around. Torricelli’s new device was the first mercury barometer. A barometer measures the amount of pressure, or weight that air pushes down from the atmosphere. Torricelli viewed the atmosphere like an ocean of water above our heads, which would have weight, and this thick atmosphere of gasses would push down against the surface. The more pressure or weight the atmosphere had, the higher the mercury would be in the glass tube.
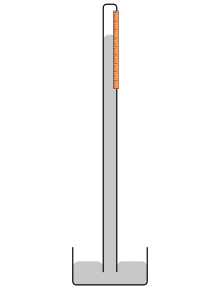

Blaise Pascal wondered if Torricelli was right, and quickly came to an idea to test it. If you climbed a gigantic mountain, the amount of atmosphere above you would be less, and hence the pressure exerted would be less as well, and the mercury in a barometer would be lower than observed at the base of the mountain. Pascal was not a mountaineer nor much of an adventurer. He did have two sisters, one of which was married to a handsome man named Florin Périer. Over the summer Pascal convinced his brother-in-law to hike to the top of the volcano, Puy de Dôme, and along the way measure the height of the mercury in the tube of a barometer. Périer agreed and in September he made the hike to the summit, recording the level of the mercury along the way. At the same time, near the base of the mountain, the level of the mercury in a second barometer was measured. It was found that as Périer hiked higher and higher up the volcano. The lower the mercury level was compared to the barometer at the base of the mountain. Pascal was delighted at the results of the experiment and proved that Torricelli was right; the atmosphere had weight that presses down on the surface of the Earth. Barometers quickly became a new scientific tool to measure atmospheric pressure (as well as the height of mountains). Atmospheric pressure decreases with increasing elevation. The higher the altitude you are, the lower the atmospheric pressure. Gasses have weight, and press down on the surface of the Earth, due to the atmosphere’s mass and the Earth’s gravitational force. Near the surface of Earth at sea level, the pressure exerted by the atmosphere is 101,325 Pascals, which is equivalent 1013.25 millibars, or 101.325 kilopascals, or equivalent to mercury measured up to 760 mm in height (often expressed as 760 mm Hg).


What happens to a helium filled balloon as it rises in the atmosphere? If there is less pressure exerted on it as it rises upward, what happens to the balloon? With less pressure, the balloon will expand the higher up it floats into the atmosphere, as the gasses inside the balloon will fill more volume, as less pressure is pushing against the outside of the balloon. Eventually the balloon will pop or break, as it is stretched larger.

You may experience discomfort when you drive quickly over a mountain pass, or are in an airplane due to this change in pressure. Inside your ears, the middle ear cavity (which holds the incus, malleus and stapes bones) is an air-filled cavity. As atmospheric pressure decreases, this middle ear cavity can expand, by pushing against your ear drum, causing discomfort. A small opening, controlled by a muscle can open this air-filled cavity to relieve this building internal pressure, but this opening can be blocked by mucus if you have a head cold or sinus infection, this condition could result in a ruptured ear-drum.
The gas molecules that compose Earth’s atmosphere are compressed by the atmospheric pressure, such that the density of gas molecules increases the closer you get to the surface of the Earth, and the closer you are to sea level. Gasses are highly compressible by the weight of the atmosphere above, and results in the highest concentration of gas molecules near the surface of the Earth. In fact, half of all gas molecules in the atmosphere reside below an altitude of 5.5 kilometers, or 18,045 feet. This means that there are nearly half as many molecules of oxygen available per volume on top of an 18,000-feet mountain, as there is near sea level. This is why mountaineers climbing high mountains, such as Everest (29,000 feet high), will take oxygen tanks with them. What causes altitude sickness is the thin mountain air that results from less atmospheric pressure and less available oxygen molecules per volume. Hiking a high mountain requires more breaths for the body to receive the same amount of oxygen compared to a hike at sea level. Measuring atmospheric pressures using a barometer is an important tool in weather forecasting. Atmospheric pressure can vary from place to place on the surface of the Earth, and over time. Isobar maps are daily to hourly maps composed of contour lines of equal atmospheric pressures, showing geographic regions of high pressure and low pressure, as the distribution of the atmosphere can change over time. The highest recorded pressure on the surface of the Earth was 108.48 kilopascals (in Mongolia) with the lowest recorded value of 87.00 kilopascals (during a 1979 typhoon in the Pacific), compared to the standard sea level atmospheric pressure of 101.325 kilopascals. Atmospheric pressures vary across the surface of the Earth and are important in weather forecasting.
| Previous | Current | Next |
|---|---|---|
