Planet Earth/3h. Mass spectrometers, X-Ray Diffraction, Chromatography and Other Methods to Determine Which Elements are in Things
The chemical make-up and structure of Earth’s materials
[edit | edit source]
In 1942, Victor Goldschmidt having escaped from Norway arrived in England, among a multitude of refugees from Europe. Shortly after arriving in London he was asked to teach about the occurrence of rare elements found in coal to the British Coal Utilisation Research Association, which was a non-profit group funded by coal utilities to promote research. In the audience was a young woman named Rosalind Franklin. Franklin had recently joined the coal research group having left graduate school at Cambridge University in 1941, and leaving behind a valuable scholarship. Her previous advisor Ronald Norris was a veteran of the Great War, and suffered as prisoner of war in Germany. So, the advent of World War II plagued him, and he took to drinking and was not supportive of young Franklin’s research interests. In his lab, however, Franklin was exposed to the methods to the chemical analysis of using photochemistry, or light to excite materials to produce photons of differing light-waves of energy.

After leaving school, her research focused on her paid job to understand the chemistry of coal, particularly how organic molecules or hydrocarbons break down through heat and pressure inside the Earth and thus also leading to decreasing porosity over time (the amount of space or tiny cavities) within the coal. Franklin moved to London to work, and stayed in a boarding house with Adrienne Weill, a French chemist and refugee who was a former student of the famous chemist Marie Curie. Adrienne Weill became her mentor during the war years, while Victor Goldschmidt taught her in a classroom during the war. With the allied victory in 1945, Rosalind Franklin returned to the University of Cambridge and defended her research she had conducted on coal in 1946. After graduation, Franklin asked Adrienne Weill if she could come to France to continue her work in chemistry. In Paris, Franklin secured a job in 1947 at the Laboratoire Central des Services Chimiques de l'État, one of the leading centers for chemical research in post-war France. It was here that she learned of the many new techniques being developed to determine the chemical make-up and structure of Earth’s materials.
What allows scientists to determine what specific chemical elements are found in materials on Earth? What tools do chemists use to determine the specific elements inside the molecules that make up Earth materials, whether they be gasses, liquids or solids?
Using Chemical Reactions
[edit | edit source]Any material will have a specific density at standard temperatures and pressures, and exhibit phase transitions at set temperature and pressures, although elucidating this from every type of material can be challenging if the material has extremely high or low temperatures/pressures for these phase transitions. More often than not, scientists use chemical reactions to determine if a specific element is found in a substance. For example, one test to determine the authenticity of a meteorite is to determine if it contains the element nickel.

Nickel as a siderophile element is rare on the surface of Earth, with much of the planet’s nickel being found in the Earth’s core. Hence, meteorites have a higher percentage of nickel than most surface rocks. To test for nickel, a small sample is ground up into a powder, and added to a solution of HNO3 (nitric acid). If nickel is present the reaction will result in ions of nickel (Ni+ and Ni2+) to be found in the solution, a solution of NH4OH (Ammonium hydroxide) is added to increase the pH of the solution by introducing OH- ions, this will cause any iron ions (Fe2+ and Fe+3) to oxidize with the OH- ions, which is a solid (and will be seen as an rust-color solid on the bottom of the solution), the final step is to pour off the clear liquid, which now should contain ions of nickel (Ni+ and Ni2+) and add Dimethyl-glyoxine (C4H8N2O2) a complex organic molecule which reacts with ions of nickel. Ni+2C4H8N2O2→Ni(C4H8N2O2)2
Nickel bis(dimethylglyoximate) is a bright red solution, and a bright red color would indicate the presence of nickel in the powdered sample. This method of determining nickel was worked out by Lev Chugaev, a Russian chemistry professor at the University of Petersburg in 1905. This type of diagnostic test may read a little like a recipe in a cookbook, but provides a “spot” test to determine the presence or absence of an element in a substance. Such tests have been developed for many different types of materials in which someone wishes to know if a particular element is present in a solid, liquid and even in gasses.
Such methodologies can also separate liquids that had been mixed together by utilizing differences in boiling temperatures, such that a mixture of liquids can be concentrated back into specific liquids based on what temperature each liquid substance boils at. Such distillation methods are used in petroleum refining processes at oil and gas refineries. The heating of liquids contain crude oil can be used to separate out different types of hydrocarbon oils and fuels, such as kerosene, octane, propane, benzene, and methane.
Chromatography
[edit | edit source]One of the more important innovations in chemical analysis is chromatography, which was first developed by the Italian-Russian chemist, Mikhail Tsvet, whose Russian surname Цвет, means color in Russian. Chromatography is the separation of molecules based on colors, and was first developed in the study of plant pigments found in flowers. The method is basically to dissolve plant pigments in a solution of ethanol and calcium carbonate, which will separate different color bands that can be observed in a clear beaker. Complex organic molecules can be separated out using this method, and purified.
Using this principle, gasses can be analyzed in a similar way through gas chromatography, which is where gasses (often solid or liquid substances that have been combusted or heated until they become a gas in a hot oven) are passed through a column with a carrier gas of pure helium. As the gasses pass by a laser sensor, the color differences are measured at discrete pressures which are adjusted within the column. Gas chromatography is an effective way to analyze the chemical makeup of complex organic compounds.
Color is an effective way to determine the chemical ingredients of Earthly materials, and it has been widely known that certain elements can exhibit differing colors in materials. Many elements are used in glass making and fireworks to dazzling effect. Pyrolysis–gas chromatography is a specialized type of chromatography in which materials are combusted at high temperatures, and the colors of the produced smaller gas molecules are measured to determine their composition. Often gas chromatography is coupled with a mass spectrometer.
-
The field of chromatography was first developed in the study of pigments in flowers and plants.
-
Exposed to a hot flame, which changes color to blue green indicates the presence of copper. Such elements are used to color fireworks.
-
Fireworks, different colors are produced by different elements.
Mass spectrometry
[edit | edit source]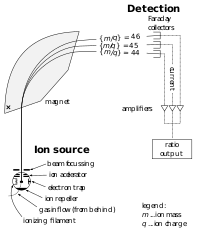
A mass spectrometer measures the differing masses of molecules and ionized elements in a carrier gas (most often inert helium). Mass spectrometry examines a molecule’s or ion’s total atomic mass by passing it through a gas-filled analyzer tube between strong magnets which deflect their path based on the atomic mass. Lighter molecule/ions will deflect more than heavier molecule/ions resulting in them striking a detector at the end of the analyzer tube at different places, which produces a pulse of electric current in each detector. The more the electric current is recorded the higher the number of molecules or ions of that specific atomic mass. Mass spectrometry is the only way to measure various isotopes of elements since the instrument measures directly the atomic mass.
There are two flavors of mass spectrometers. The first is built to examine isotopic composition of carbon, oxygen, as well as nitrogen, phosphorus and sulfur. Elements common in organic compounds, but which combust in the presence of oxygen, producing gas molecules of CO2, NO2, PO4 and SO4. These mass spectrometers can be used in special cases to examine the hydrogen and oxygen isotopic composition in H2O. They are also useful to measure carbon and oxygen isotopes in calcium carbonate CaCO3, when reacted with acid producing CO2. The other flavor of mass spectrometers ionizes elements (remove electrons) under extremely high temperatures, allowing ions of much higher atomic mass to be measured through an ionized plasma beam. These ionizing mass spectrometers can measure isotopes of rare transitional metals, such as nickel (Ni), lead (Pb), and uranium (U), for example the ratios used in radiometric dating of zircons. Modern mass spectrometers can laser ablate tiny crystals or very small materials using an ion microprobe, capturing a tiny fraction of the material to pass through the mass spectrometer, and measure the isotopic composition of very tiny portions of substances. Such data can be used to compare the composition across surfaces of materials at a microscopic scale.

The first mass spectrometers were developed during the 1940s, after World War II, but today are fairly common in most scientific labs. In fact, the Sample Analysis at Mars (SAM) instrument on the Curiosity Rover on the surface of Mars contains a gas chromatograph coupled with a mass spectrometer, allowing scientists at NASA to determine the composition of rocks and other materials encountered on the surface of Mars. Rosalind Franklin did not have access to modern mass spectrometers in 1947, and their precursors, gigantic scientific machines called cyclotrons, were not readily available in post-war France. Instead, Rosalind Franklin trained on scientific machines that use electromagnetic radiation to study matter, using specific properties of how light interacts with matter.
Using Light
[edit | edit source]The major benefit to using light (and more broadly all types of electromagnetic radiation) to study a substance, is that using light does not require that the bonds between atoms be broken or changed in order to study them. So, these techniques using light do not require that a substance be destroyed by reacting it with other chemicals or altering it by combustion to determine its composition as a gas.


Earlier crystallographers, who studied gems and jewels noticed the unique ways in which materials would absorb and reflect light in dazzling ways, and to understand the make-up of rocks and crystals, scientists used light properties or luminosity to classify these substances. For example, minerals composed of metallic bonds, will exhibit a metallic luster or shine and are opaque, while covalently and ionically bonded minerals are often translucent, allowing light to pass through. The study of light passing through crystals, minerals and rocks to determine the chemical make-up crystals is referred to as petrology. More generally, petrology is the branch of science concerned with the origin, small-scale structure, and composition of rocks and minerals and other Earth materials under a polarizing light microscope.

Refraction and Diffraction of Light
[edit | edit source]Light in the form of any type of electromagnetic radiation interacts with material substances in three fundamental ways: The light will be absorbed by the material, bounce off the material, or pass through the material. In opaque materials, like a gold coin, light will bounce off the material, or diffract from the surface, while in translucent materials, like a diamond, light will pass through the material and exhibit refraction. Refraction is how a beam of light bends within a substance, while diffraction is how a beam of light is reflected off the surface of a substance. They are governed by two important laws in physics.
Snell's Law of Refraction
[edit | edit source]Snell’s law is named after Ibn Sahl, a Persian scientist who published his early thesis on mirrors and lenses in 984 CE. Snell’s law refers to the relationship between the angle of incidence and the angle of refraction resulting from the change in velocity of the light as it passes into a translucent substance. Light slows down as it passes into a denser material. By slowing down, the light beam will bend, the amount that the beam of light bends is mathematically related to the angle that the beam of light strikes the substance and the change in velocity.

The mathematical expression can be written where the angle from perpendicular to the substance the light strikes the outer surface of the substance, and is the angle from perpendicular that the light bends within the substance. v1 is the velocity of light outside the substance, while v2 is the velocity of light within the substance.
Some translucent materials reduce the velocity of light more than others. For example, quartz (SiO2) exhibits high velocity, such that the angle of refraction is very low. This makes materials made of silica, or SiO2, such as eye glasses and windows easy to see through.

However, calcite (CaCO3) also known as calcium carbonate, exhibits very low velocities, such that the angle of refraction is very high. This makes light bend within the materials made of calcite. Crystals of calcite are often sold in rock shops or curio shops as “Television” crystals, because the light bends so greatly as to make any print placed below a crystal appear to come from within the crystal itself, like the screen of a television. Liquid water with a lower velocity than air, also bends light, resulting in the illusion of a drinking straw appearing broken in a glass containing water.
Measuring the angles of refraction in translucent materials can allow scientists to determine the chemical make-up of the material, often without having to destroy or alter the material. However, obtaining these angles of refractions often requires making a thin section of the material to examine under a specialized polarizing microscope designed to measure these angles.
Bragg's Law of Diffraction
[edit | edit source]Bragg’s law is named after Lawrence Bragg and his father William Bragg, who both discovered the crystalline structure of diamonds, and were awarded the Nobel Prize in 1915 for their work on diffraction. Unlike Snell’s law, Bragg’s law results from the inference of light waves as they diffract (reflected) off a material’s surface. The wave length of the light needs to be known, as well as the angle that the light is reflected from the surface of the substance.

The mathematical expression can be written where the light wavelength is λ, and is the angle from the horizontal plane of the surface (or glancing angle), and d is the interplanar distance between atoms in the substance or the atomic-scale crystal lattice plane.
The distance d can be determined if λ and are known. n an integer, which is the “order” of diffraction/reflection of the light wave.
Monochrome light (that is light of only one wave length) is shone onto the surface of a material at a specific angle, resulting in light reflected off the material which is measured at every angle from the material. Using this information, the specific distances between atoms can be measured.
Since the distance between atoms is directly related to the number of electrons within each orbital shell, each element on the periodic table will have different values of d, depending on the orientations of the atomic bonds. Furthermore, different types of bonds of the same elements will result in different d distances. For example, both graphite and diamonds are composed of carbon atoms, but are distinguished from each other in how those atoms are bonded together. In graphite, the carbon atoms are arranged into planes separated by d-spacings of 3.35Å, while in diamonds the d-spacings are closely together linked by covalent bonds of 1.075Å, 1.261Å, and 2.06Å distances.
-
Diamond is made of atoms of carbon, but the d-spacings are closely together linked by covalent bonds of 1.075Å, 1.261Å, and 2.06Å distances.
-
Graphite is also made of atoms of carbon, but the atoms are arranged in planes separated by d-spacings of 3.35Å
These d-spacings are very small, requiring light with short wavelengths within the X-ray spectrum with a wavelength (λ) of 1.54Å. Since most atomic bonds are very small, X-ray electromagnetic radiation is typically used in studies of diffraction. The technique used to determine d-spacings within materials is called X-Ray Diffraction (XRD). It often is coupled with tools to measure X-ray fluorescence (XRF), which measures the energy states of excited electrons that actually absorb X-rays and release energy as photons. X-Ray Diffraction measures how light wave reflect off the spacing between atoms, while X-Ray Fluorescence measures how light waves are emitted from atoms which were excited by light striking the atoms themselves. Fluorescence looks at the broad spectrum of light emitted, while Diffraction only looks at monochromic light (light of a single wave-length).
Great advances have been made in both XRD and XRF tools, such that many hand-held analyzers now allow scientists to quickly analyze the chemical make-up of materials outside of the laboratory and without destruction of the materials understudy. XRD and XRF has revolutionized how materials can be quickly analyzed for various toxic elements, such as lead (Pb) and arsenic (As).
However, in the late 1940s, X-Ray diffraction was on the cutting edge of science and Rosalind Franklin was using it to analyze more and more complex organic molecules – molecules containing long chains of carbon bonded with hydrogen and other elements. In 1950, Rosalind Franklin was awarded a 3-year research fellowship from an asbestos mining company to come to London to conduct chemistry research at King’s College. Staffed with the state-of-the-art X-Ray diffraction machine, Rosalind Franklin set to work to decode the chemical bonds that form complex organic compounds found in living tissues.
The Discovery of the Chemistry of DNA
[edit | edit source]She was encouraged to study the nature of a molecule called deoxyribonucleic acid, or DNA that was found inside living cells particularly sperm cells. For several months she worked on a project to unravel this unique molecule, when one day an odd little nerdy researcher by the name of Maurice Wilkins arrived at the lab. He was furious with Rosalind Franklin, as he came having been away on travel, but before leaving had been working on the very topic Rosalind Franklin was working on, using the same machine. The Chair of the Department had not informed either of them of their research work and that they were to be sharing the same equipment and lab space. This, as you can imagine, caused much friction between Franklin and Wilkins. Despite this setback Rosalind Franklin had made major breakthroughs in the few months she had access to the machine alone, and was able to uncover the chemical bonds found in deoxyribonucleic acid. These newly deciphered helical-bonds allowed the molecule to spiral like a spiral stair-case. However, Franklin was still uncertain. During 1952 both Franklin and Wilkins worked alongside each other, on different samples, and using various techniques, using the same machine. They also shared a graduate student who worked in the lab between, Raymond Gosling. Sharing a laboratory with Wilkins continued to be problematic for Rosalind Franklin and she transferred to Birkbeck College in March of 1953, which had its own X-Ray diffraction lab. Wilkins returned to his research on deoxyribonucleic acid using the X-Ray diffraction at Kings College, however a month later, in 1953 two researchers at Cambridge University, announced to the world their own solution to the structure of DNA in a journal article published in Nature.

Their names were Francis Crick and James Watson. These two newcomers published their solution before either Franklin and Wilkins had a chance too. Furthermore, they both had only recently began their quest to understand deoxyribonucleic acid, after being inspired by attending scientific presentations by both Franklin and Wilkins. Crick and Watson lacked equipment, so they spent their time using models by linking carbon atoms (represented by balls) together to form helical towers with other elements. Their insight came from a famous photograph taken by Franklin and her student Gosling, and was shown to them by Wilkins in early 1953. Their research became widely celebrated in England, as it appeared that the American scientist Linus Pauling was close to solving the mystery of DNA, and the British scientists had uncovered it first.
In 1962, Wilkins, Crick and Watson shared the Nobel Prize in Physiology and Medicine. Although today Rosalind Franklin is widely recognized for her efforts to decipher the helical nature of the DNA molecule, she died of cancer in 1958 before she could be awarded a Nobel Prize. Today scientists can map out the specific chemistry of DNA, such that each individual molecule within different living cells can be understood well beyond its structure. Understanding complex organic molecules are of vital importance in the study of life on planet Earth.
Rayleigh and Raman scattering
[edit | edit source]
There stands an old museum nestled in the bustling city of Chennai along the Bay of Bengal in eastern India, where a magnificent crystal resides on a wooden shelf accumulating dust in its display. It was this crystal that would excite one of the greatest scientists to investigate the properties of matter, and discover a new way to study chemistry from a distance. This scientist was Chandrasekhara Venkata Raman of India, often known simply as C.V. Raman. Raman grew up in eastern India with an inordinate fascination for shiny crystals and gems and the reflective properties of minerals and crystals. He amassed a large collection of rocks, minerals, and crystals from his travels. One day he purchased from a farmer a large quartz crystal which contained trapped inclusions of some type of liquid and gas inside the crystal. The quartz crystal intrigued him, and he wanted to know what type of liquid and gas was inside. If he broken open the crystal to find out, it would ruin the rarity of the crystal, as such inclusions of liquid and gasses inside a crystal are very rare. Without breaking open the crystal, if Raman used any of the techniques described previously, he could only uncover the chemical nature of the outer surface of the crystal, likely as silica dioxide (quartz).
To determine what the liquid and gas were inside, he set about inventing a new way to uncover the chemical make-up of materials that can only be seen. His discovery would allow scientists to not only know the chemical make-up inside this particular crystal, but allow scientists to determine the chemical make-up of far distant stars across the universe, tiny atoms on the surfaces of metals, as well as gasses in the atmosphere.
Light has the unique ability to reveal the bonds within atomic structures without having to react or break those bonds. If a material is transparent to light, then it can be studied. Raman was well aware of the research of Baron Rayleigh, a late nineteenth century British scientist who discovered the noble gas argon by distilling air to purify it. Argon is a component of the air that surrounds you, and as an inert gas unable to bond to other atoms it exists as single atoms in the atmosphere. If a light bulb is filled with only purified argon gas, light within the bulb will pass through the argon gas producing a bright neon-like purple color. If a light bulb is filled with helium (He) gas, it would produce a bright reddish color, but the brightest light would be seen with neon (Ne), a bright orange color. These noble gasses produced bright neon-colors in light bulbs and soon appeared during in the early twentieth-century as bright neon window signs in store fronts of bars and restaurants.

Why did each type of gas result in a different color in these lightbulbs? Rayleigh worked out that the color was caused by the scattering of light waves due to the differences in the size of the atoms. In the visual spectrum of light, light waves are much larger than the diameter of these individual atoms, such that the fraction of light scattered by a group of atoms is related to the number of atoms per unit volume, as well as the cross-sectional area of the individual atoms. By shining light through a material, you could measure the scattering of light and in theory determine the atom’s cross-sectional area. This Rayleigh scattering could also be used to determine temperature, since with increasing temperature the atoms enlarged in size.
In addition to Rayleigh scattering the atoms also absorb light waves of particular wave lengths, such that a broad range of light wavelengths of light passed through a gas would be absorbed at discrete wavelengths, allowing you to fingerprint certain elements within a substance based on these spectral lines of absorption.
In his lab, Raman used these techniques to determine the chemical make-up of the inclusions within the crystal, by shining light through the crystal. This is referred as spectroscopy, the study of the interaction between matter and electromagnetic radiation (light). Raman did not have access to modern lasers, so he used a mercury light-bulb and photographic plates to record where light would be scattered, as thin lines appeared where the photographic plate was exposed to light. It was time consuming work, but eventually led to the discovery that some of the scattered light had lost energy and shifting into longer wave lengths.
Most of the observed light waves would bounce off the atoms without any absorption (elastic) or Rayleigh Scattering, while other light waves would be fully absorbed by the atoms. However, Raman found that some light waves would both bounce off the atoms and contribute some vibrational energy to the atoms (inelastic) energy, which became known as Raman Scattering. The amount of light that would scatter and be absorbed was unique to each molecule. Raman discovered a unique way to determine the chemistry of substances by using light, which today is called Raman spectroscopy, a powerful tool to determine the specific chemical makeup of complex materials and molecules by looking at the scattering and absorption of light. In the end, CV Raman determined that the mysterious fluid and gas within the quartz crystal was water (H2O) and methane (CH4). Today, the crystal still remains intact in a Bangalore museum, residing in the institute named after Raman, the Raman Research Institute of India.
Scientists today have access to powerful machines to determine the chemical make-up of Earth’s materials to such an extent that nearly any type of material, from solids, liquids and gasses and even plasma, can be determined as to which elements are found within the substance under study. Each technique has the capacity to determine the presence or absence of individual elements, as well as the types of bonds that are found between atoms. Now that you know a little of how scientists determine the makeup of Earth materials, we will examine in detail Earth’s gasses, in particularly Earth’s atmosphere.
| Previous | Current | Next |
|---|---|---|








