Planet Earth/2c. Electromagnetic Radiation and Black Body Radiators
Color and Brightness
[edit | edit source]
During the 1891-92 academic year, a young woman named Henrietta Leavitt enrolled in a college class on astronomy, and it changed her life. Her fascination with stars was ignited during the class. A class that was only offered to her due to the Society for the Collegiate Instruction for Women at the Harvard Annex, and at a time when women were not allowed to enroll at the main Harvard University. Leavitt, in her final year, was left curious after earning an A−, with an ambitious eagerness to study the stars as a full-time profession. After the class, and even after she had graduated college, she began volunteering her time at the Harvard University observatory, organizing photographic plates that were taken of stars nightly observed using the new high-power telescope at the university. The photographic plates were being used by researchers to catalogue stars, noting their color and brightness.

Astronomers were very interested in measuring the distance from Earth to these stars observed in the night sky. Scientists had known for many years the distance to the moon and sun, by measuring something called parallax. Parallax is the effect where the position of an object appears to differ when viewed from different observational positions. For example, closing one eye, hold up your thumb and take a sighting with your thumb, so that your thumb lines up to an object far away. If you were to switch eyes, you will notice that the far away object jumps to a different position relative to your thumb. Using some basic Math, you can calculate how far away the object is from you, as the closer the object is, the more it will change position based on your observational point of view. However, when distances are very far, the difference in the positions from different observation points on Earth are so small relative to the distance to the object that they cannot be measured. Stars were just too far away to measure the actual distance from Earth, and scientists were eager to learn the size and dimensions of the universe.
Henrietta Leavitt’s journey to discover a tool to measure these stellar distances, was a lengthy one. Although she begun working on a report describing her observations, she was interrupted with travel to Europe, and a move to Wisconsin, where rather than teaching science, she got a job teaching art at Beloit College. Her experience in Wisconsin, and the cold climate, resulted in her becoming very ill, and she lost her ability to hear. Left deaf for the rest of her life with the illness, she wrote back to Harvard about gaining employment there to help organize and work on the photographic plates of stars, a pursuit that still interested her. She returned to her work, which resulted in a remarkable discovery.
Astronomers measure what is called the Apparent Magnitude of stars by measuring a star’s brightness. Large stars far away will have equal brightness as closer smaller stars, as it was impossible to tell distances to stars and determine a star’s Absolute Magnitude. The apparent magnitude of a star was measured on the photographic plates taken by the observatory telescope, but Henrietta Leavitt observed a strange relationship when she looked at a subset of the 1,777 stars in her catalogue. She looked at 25 stars located in the small Magellanic cloud, that were believed to be roughly the same distance from Earth. These stars were in a cluster and close together. Furthermore, these 25 stars were recognized as cepheid variable stars, which are stars that pulse in brightness over the course of several days to weeks.

Henrietta Leavitt carefully measured the brightness of these stars for days to weeks, and determined the periodicity of the pulses in brightness, and found that the brighter the star, the longer the periodicity of its pulses. Since these stars were roughly the same distance from Earth, this relationship indicated a method of how you could tell how far away a star was from Earth, by looking at the periodicity of the pulses in brightness. If two stars had equal brightness, but one had a longer periodicity between pulses of brightness, the star with the shorter periodicity between pulses would be closer. Hence, Henrietta Leavitt discovered a yardstick to measure the universe. She published her findings in 1912, in a short 3-page paper, dictated to her supervisor Edward Pickering. Her discovery would come to importance later, but first you should learn what light really is.
What is Light and Electromagnetic Radiation?
[edit | edit source]What is light? For artists, light is a game of observation, as without it, there is no way of seeing, only darkness. Historically light was seen as a construction of the mind, of how your eyes take in your surroundings, but centuries of experiments show that light is caused externally by the release of energy into the surrounds. A very good analogy for the concept of light is to imagine a ball that is rolling up and down over a hill as it travels. Using Noether’s theorems we can suggest that this ball is oscillating between a position at the top of the hill, where the energy is stored as potential energy, and at the bottom of the hill where the energy has been released as kinetic energy causing the ball to then rise up over the next hill. Since it travels at the speed of light, the ball never loses energy by entropy as it rises up the next hill.
The name for this traveling mass-less ball is a Photon, and the distance between the hills is called the Wave Length. Hence light can be viewed as both a particle and wave. The hills, or wave lengths can be oriented up and down, side to side or diagonally in any orientation to the path of travel for the Photon. Polarized light is where the orientation is limited to a single direction.


If you ever seen a modern 3D movie in a theatre, film makers use polarizing lenses in 3D glasses to project two sets of images at the same time, the right eye has the light oriented in one direction, while the left eye has the light oriented in another direction (often perpendicular). So the blurry movie image can be broken into two separate images for each eye at the exact same time, making an illusion of dimensionality. If you cut out the two lenses in your 3D glasses, you can orient them perpendicularly to each other so one lens allows only vertical oriented light waves while the other allows only horizontal oriented light waves, resulting in darkness.
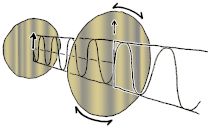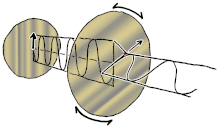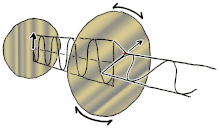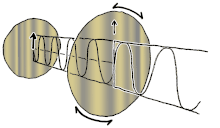

This is called cross-polar as no light can pass. However, you can place a crystal or lens between the two lenses of polarizing light, which can bend or reflect the light in a different orientation, doing so will allow some light waves to bend or change orientation between the two lenses and allowing light to then travel through the previous black lens, this is called birefringence. Birefringence is an optical property of a material having a refractive index that depends on the polarization and propagation direction of light. It is an important principle in crystallography and has resulted in the break-throughs in liquid crystal displays (LCD) flat-panel television displays that are found in proliferation hung on walls of sports-bars, airports and living rooms around the world. Different voltages can be applied to each liquid crystal layer representing a single pixel on the screen. This voltage shifts the birefringence of the crystal, allowing light to pass through the top polarizing lens that previously blocked the light. Color can be added with color filters. Hence, if you are reading these words on an electronic LCD, then it is likely due to this bending of the orientations of polarized light allowing you to do so.
Wavelength of Light and Color
[edit | edit source]
The photon particle travels at the maximum speed of light or very near the maximum speed of light, but can have differing amounts of energy, based on the distance of the wave-length. A photon bouncing over shortly spaced steep hills has more energy, than a photon that bounces over distantly spaced gently sloping hills. Using this analogy, light behaves both as a particle and a wave. This was first demonstrated by Thomas Young (the polymath that translated Egyptian Hieroglyphs, and coined the word Energy) in 1801, who placed two slits in some paper, and shone a light through them, demonstrating a strange pattern on a screen as the light waves interacted with each other, causing there to be a pattern of interference in the light projected on a screen. Similar to the ripples seen in a pond, when two rocks are dropped into the water. This interference is caused by the two beams of light waves intersecting with each other.

Light can be split into different wave-lengths by use of a prism, the resulting rainbow of colors is called a spectrum. A spectrum separates light of diffing wave-lengths, rather than orientations, resulting in light bands of different colors. A rainbow is a natural feature caused by rain drops acting as a prism to separate out the visible colors of light.

Normal sunlight looks white, but is in fact a mix of light traveling over differing wavelengths, light purple light travels over the shortest wavelengths, with an average wavelength of 400 nm (1 nm = 0.000000001 meters or 1x10-9 meters), while dark red travels of the longest wavelengths, with an average of 700 nm wavelength. The mnemonic ROY G. BIV for the colors in the visible light spectrum is a helpful mnemonic for the order of colors from longest to shortest wavelength. Red, Orange, Yellow, Green, Blue, Indigo, Violet, with Red having the longest wavelength, hence least amount of energy, while Violet (or Purple) having the shortest wavelength and hence most amount of energy. Light can travel along wavelengths that are both above and below these values, this special “invisible” light is collectively called Electromagnetic Radiation, which refers to both visible and non-visible light along the spectrum.
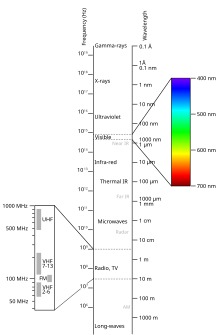
Sunlight
[edit | edit source]Sunlight contains both visible and non-visible light, and hence scientists call this energy the Sun’s Electromagnetic Radiation. Infra-Red light is light with a longer wavelength than visible light, while Ultra-Violet light is light with a shorter wavelength than visible light. Ultra-Violet or (UV) light contains more energy, and can with prolonged exposure cause sun burns, and eventually skin cancer. Sunscreen blocks this higher energy light from hitting the skin, and UV sunglasses block this damaging light from hitting the eye, causing cataracts. The lower energy Infra-Red light is important in the development of “night-vision” googles, as these glasses shift low energy Infra-Red light into the visible spectrum. This is useful for Thermo-imaging, as warmer objects will exhibit shorter wave-lengths of Infra-Red light, than colder objects. The most highly energized light on the spectrum of electromagnetic radiation is gamma rays. This very short wavelength electromagnetic radiation is the type of light that first emerges from nuclear fission in the Sun’s core. Gamma rays have so much energy, that they can pass through solid matter. While often invoked in Comic Books as the source of super powers, Gamma rays are the most dangerous form of electromagnetic radiation, in fact this “radiation” from nuclear fusion and fission results in a form of light, which can pass through materials, such as the tissues of living animals and plants, and in doing so seriously damage the molecules in these life forms resulting in illness and death. Slightly lower, but still highly energetic electromagnetic radiation, are the short-wavelength X-rays, which are also known for their ability to pass through material, and used by doctors to see your bones. X-Rays can also be damaging to living tissue, and prolonged exposure can cause cancer, and damage to living cells. Nuclear radiation is the collective short-wave electromagnetic radiation of both gamma and X-rays, which can pass through materials, and are only stopped by material composed of the highest mass atoms, such as lead. The next type of Electromagnetic radiation is the slightly longer wavelengths of Ultra-Violet light, followed by the visible light that we can see, which is a very narrow band of light waves. Below visible light is Infra-Red, which is light that has less energy than visible light, and given off from objects that are warm. Surprisingly, some of the longest wave length electromagnetic radiation are microwaves, which are below Infra-Red, with wave lengths between 1 and 10 centimeters. Microwaves were developed in radar communications, but it was discovered that they are an effective way to heat water molecules that are bombarded with electromagnetic radiation at this wavelength at large amplitudes. If you are using the internet wirelessly on WiFi your data is being sent to your computer or tablet over wavelengths of about 12.5 centimeters, just below the microwave frequency, and within the longest wavelength range of electromagnetic radiation— radio waves. Radio waves can have wave lengths longer than a meter, which means that they carry the lowest amount of energy along the scale of electromagnetic radiation.
Wavelength of Light, Energy and how you see the World
[edit | edit source]There is an important consideration to think about regarding the relationship between wave length and the amount of energy that a photon carries. If the wavelength is short, the photon has to travel a farther absolute distance than a photon traveling with a longer wavelength, which travels a path that is straighter. Light waves are like observing two racing cars that complete the race at the exact same time, but one of the racing cars had to take a more winding path, than the other. Light only shifts into a longer wavelength and reduces its energy when it interacts with mass, the more mass the light wave impacts, the more reduced its energy will be, and the longer the resulting wavelength. This is how you observe the universe, how you see! Photons when they collide with mass, shift into a longer wavelength and exhibit less energy, some of this energy is transferred to the atoms resulting in heat. This shift in wavelength causes anything with sufficient mass to reflect light that is of different colors and shades, by altering the wavelength.
Color Systems
[edit | edit source]
Color is something that every artist understands, but the modern science of color emerged with a painting of one of the most famous blind individuals in American history, Helen Keller. Helen Keller was born with sight and hearing, but quickly lost both as a baby when she fell ill. Locked in darkness and silence for the rest of her life, she learned how to communicate through the use of her hands, using touch. Later she authored many books, and went on to promote equal rights for women. Her remarkable story captured a great amount of international interest among the public, and a portrait was commissioned of her by an artist named Albert H. Munsell in 1892. Munsell painted an oil painting of Helen Keller, which hangs in the American Foundation for the Blind, and the two became good friends. The impression likely had a lasting effect on Albert Munsell, as he began research on color shortly afterward in an attempt to understand color, more as a curious scientist rather than as an artist. Focusing on landscape art, Munsell likely understood a unique method artists employ to limit light when trying to capture a bright land or sea scape. This is done by holding a plate of red glass in front of the view that is to be painted. Because red has the longest wavelength of the visual spectrum, the wavelengths are shifted to longer lengths beyond our ability to see, lower wavelengths become so low they become darkened or absent (as infra-red), while brighter light results in just red visible light. Hence the value of the light can be rendered much easier in a painting or drawing.
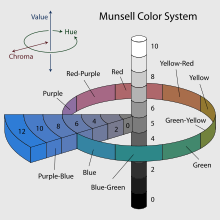
Munsell began to classify color by its grayness, on a scale from 0 as pure black to 10 as pure white, with various shades of gray between these values. This measure of color is called value, and could be seen among all colors if a red filter was placed over them, or in modern way, by taking a black and white photograph of the colors, the observable difference of color is lost, but the value of the color is retained. For example, if a deep rich yellow paint has the same value as a bright red paint, under a black and white photograph the two colors would look identical. Hue was the named color; red, yellow, green, blue, purple and violet, and represents the wavelength of the visible spectrum. The last classification of color, was something Munsell called Chroma. Chroma is how intense the color is, for example a color with high chroma would be neon-like or very bright and annoying. These high-chroma colors are caused by light waves that have a higher amplitude in their wavelengths. Amplitude is a measure of how high or tall the light waves are, which is another parameter that light has, in addition to wavelength, energy and orientation.
Albert Munsell was impressed by his new classification of color, and set about educating 4th to 9th graders in Boston on his new color theory, as a new elementary school art curriculum. Munsell’s color classification had a profound effect on society and industry, as a new generation of students were taught about color from an early age. His classification of color resulted in a profound change in fashion, design, art, food, cooking, and advertising. But his color science also had a profound effect on a Henrietta Leavitt, at Harvard. Albert Munsell was invited by Edward Pickering to give a talk to the women astronomers under his supervision.

Although, Leavitt did not hear Albert Munsell’s talk, as she had lost her hearing by this time, she undoubtedly saw his color classification, and may have realized the importance in the difference between hue (the wavelength of light) and chroma (the amplitude or brightness of light). It was shortly afterward that she published her famous 1912 paper, which found a relationship between brightness (Apparent Magnitude) of stars and their periodicity. This paper sent shockwaves through the small astronomical community as it offered a yard-stick to measure the universe.
Astronomers were eager to attempt to measure distances to the stars using this new tool. Early attempts yielded different distances, however. One of the first systematic attempts was offered by Harlow Shapley director of the Mount Wilson observatory in Southern California. Using this yard-stick he estimated that the universe was about 300,000 light years distant from the Earth, much larger than previous estimates, but still rather small compared to modern estimates today. He viewed that the stars in the night sky were all within the Milky Way Galaxy, not all astronomers agreed with him, some viewed the Milky Way Galaxy as an island, among a sea of many other galaxies in the universe. Soon afterward, Harlow Shapley joined Henrietta Leavitt at Harvard, after the death of Edward Pickering. This left the Mount Wilson Observatory back in California in the hands of a handsome young astronomer named Edwin Hubble.
Using Light to Measure the Expansion of the Universe
[edit | edit source]
Edwin Hubble was a star athlete in track and field in high school, and played basket-ball in college, leading the University of Chicago in its first conference title. After college he was awarded a Rhode Scholarship to go to Oxford, England, to study law. Upon his return, Edwin Hubble found a job teaching high school Spanish, physics and math, as well as coaching the high school basketball team, but after his father’s death, Edwin Hubble returned to school to pursue a degree in astronomy at the University of Chicago. In 1917, war broke out, and Hubble joined the Army serving in Europe during World War I.

Returning to the United States, Hubble got a job at the new Mount Wilson Observatory in California, where later he took over after the departure of Harlow Shapley. He continued to focus on Cepheid variable stars, hoping to better measure the universe, using the tool that Henrietta Leavitt had invented. Hubble focused his attention on a star in the Andromeda spiral nebulae, he named V1 in 1923. Over weeks he observed the shift in brightness of the star measuring the periodicity which he determined was 31.4 days between maximum brightness. Using this measurement, he estimated that the distance to the Andromeda spiral nebula was over 1,000,000 light years away, a galaxy beyond our own galaxy. He wrote to Shapley, who responded to a colleague “Here is the letter that destroyed my universe.” It did not destroy a universe, rather Edwin Hubble demonstrated a much much larger universe than ever imagined, filled with other galaxies like the Milky Way. The diameter of the universe is today estimated at an astonishing large distance of 93,000,000,000 or 93 billion light-years!
But Edwin Hubble’s greatest discovery was not just the vastness of the universe, but that it was expanding at an incredible rate. This discovery was made by examining the spectrum of light waves from star light.
Black Body Radiators
[edit | edit source]
In a dark forest somewhere on Earth is a fire burning in the center of a ring of stones, and a group of humans organized around the flames. Fire has come to define what it means to be human, with its emergence so early in human history, even prior to the origin of our species, about 1 million years ago, at a time when Homo erectus ventured out of Africa and beyond. If you have ever observed the flames of a fire, you will note the shifting colors, the yellow, reds, and deep in the hot embers the blues, and possibly violets. These shifting colored flames represent the cascade of electromagnetic radiation emitted by fire that heats the surrounding air, and provides light on a dark night. The color of the flames can directly tell us the temperature of the flames, as the shorter the wave length of light emitted, the hotter the flame will be. We can also tell how hot stars are by the careful study of the color of the light spectrum they emit.

If a blacksmith places a black iron ball into a fire, they will observe changing colors as the iron ball is heated. The colors start with a black iron ball that will slowly start to glow a deep reddish color, then a brighter yellow, at even hotter temperatures the iron will glow greenish-blue and at super-heated temperatures will take on light purplish color. Examining a spectrum of colors emitted from the “Black body” radiating iron ball will demonstrate a trend toward shorter wavelengths of light emitted from the ball, as the ball is heated in the fire. A black-body is an idealized object that emits electromagnetic radiation when heated or cooled (it also absorbs this light as well).

The spectrum of light given off by the heated iron ball or “black body radiator” can be used to calculate its temperature. The same method can be used to calculate the temperature of stars, including the temperatures previously mentioned for the sun’s surface temperature (5,778 Kelvin). There is no need to take a thermometer to the hot surface of the sun, we can measure its temperature using the sun’s own light. We can also measure the temperatures of stars millions of light years away, using the same principle. The study of the spectrums of electromagnetic radiation is called Spectroscopy. In Germany during the 1850s, a scientist named Gustav Kirchhoff was fascinated with the spectrum of electromagnetic radiation given off by heated objects, and coined the term “black-body” radiators in 1862. Kirchhoff was curious what would happen if he heated or excited with electricity gas particles, rather than solid matter, like an iron ball. Would the gas glow through the same spectrum of light as it was heated? Experiments showed that the gas would give off a very narrow spectrum of wavelengths. For example, a sealed glass jar with the gas neon, would produce bright bands of red and orange light, while argon could produce blue, among other wavelengths of colored light, gases of mercury a more bluish white. These gas-filled electric lights were developed commercially into neon-lighting and fluorescent lamps, with a wide variety of spectrums of color at very discrete wavelengths.

Kirchhoff conducted a series of experiments where a solid black body was heated, in a chamber of a purified gas, and noted that in the spectrum of the light that was not allowed to pass through the gas was the same wavelengths that were emitted when the gas was heated. When these wavelengths of light are absorbed by a gas, they leave behind discrete lines in the observed spectrum. Depending on the gas particles the light traveled through the resulting spectrum of absorbed light waves were unique to each type of gas. Astronomers, such as Edwin Hubble observed within a star’s spectrum of light similar absorption lines.

This proved to be a method to determine the composition of a star. For example, this is how we know that the sun is composed of mostly hydrogen and helium, the absorption lines for those gases are indicated in the spectrum of the sun’s light. Working in Kirchhoff’s lab was a young scientist named Max Planck who wondered why objects heated up at very high temperatures seemed not to decrease wavelength indefinitely. After conducting experiment after experiment, Max Planck determined a value to convert electromagnetic radiation wavelength to a measure of energy. This special value became known as Planck’s constant h. Currently {{{1}}} per Hertz, such that
Where E is the energy produced by the electromagnetic radiation, h is Planck’s constant, c is the speed of light, and λ is the wavelength. Note that as a function of this equation—as the wavelength increases, energy decreases. Planck’s constant is a very important number in physics and chemistry, because it relates to the size of atoms, and the distances of electrons’ orbits in the nucleus of atoms, as such Planck’s constant is also important in quantum physics. The importance of this equation is that it allows for the direct comparison between a light’s wave length and energy. Realize that energy is a measurement of the vibrational forces within particles, in other words a measurement of heat.
Fundamentally it is important to remember that electromagnetic radiation (both visible and non-visible light) is an effective way to transport energy across space. The energy within electromagnetic radiation is released as heat when electromagnetic radiation impacts particles with mass. When this happens, the electromagnetic radiation increases the length of its wavelength while transferring some of its energy into the particles. The particles increase their vibrational motion (a measure of heat). This fundamental concept explains how the Earth receives nearly all its energy, through the bombardment of light from the Sun. The Earth also receives some energy through the release of electromagnetic radiation due to the decay of radioactive atoms, which first formed during the explosive Supernova event, but have ever since been decaying. Hence electromagnetic radiation is produced by nuclear fission and fusion, but that is not the only method to produce electromagnetic radiation.
Glowing rocks or fluorescence
[edit | edit source]
In most natural history museums, there is a dark room hidden away with a display of various ordinary looking rocks. These assembled rocks however are subjected to a daily cycle of the room’s lights turning on and off, but what draws the public’s attention to these rocks, is when the room is plunged into darkness – the rocks glow. This glow is called fluorescence, and it is caused by the spontaneous production of electromagnetic radiation in the form of photons. When light waves, or any type of electromagnetic radiation impacts an atom, especially an atom that is fixed in place by its bonding in solid matter, the energy transfer from the incoming light rather than resulting in an increase in vibrational energy (heat), is instead converted into the electron field, resulting in an increase in the electron energy state. Over time, and sometimes over extraordinary long periods of time, the electron will spontaneously drop to a lower energy state, and when it does so it will release a photon. If enough atoms are affected by the incoming radiation, the dropping electron states will release enough photons to be seen in the visual spectrum, and the rock will appear to glow. Note that the incoming wavelengths of light will have to carry energy levels above the visual spectrum, and it is often UV-light that is used, but could be even shorter wavelengths of electromagnetic radiation.
When you see a rock’s fluorescence, it is the release of these photons from the drop of electron states after the electrons have moved into higher energy states when subjected to small wavelength electromagnetic radiation, such as UV-light, X-rays, and even Gamma rays. In fact, the reason radioactive material glows, is due to the release of electron energy states of the surrounding material that is subjected to the high energy and small wavelength electromagnetic radiation these radioactive materials produce.
There are a number of other ways to excite electrons into higher energy states, that can cause the spontaneous release of photons. When an object is subjected to electromagnetic radiation, scientists called the spontaneous release of photons, phosphorescence. When the object is subjected to heat or an increase in temperature, this is called thermoluminescence, for example the glow of the “black body” radiator or iron ball is an example of thermoluminescence, and is caused by the electrons dropping energy states and releasing photons, when subjected to increasing heat. The final type of fluorescence is triboluminescence which is caused by motion, or kinetic energy. Triboluminescence is found when two rocks, such as rock containing quartz are smacked together, the resulting flash of light is due to electron energy states jumping and dropping quickly releasing photons. When electrons are free from the polar attraction of protons in atoms, these free electrons are called electricity, and their motion produces photons, seen in the electric sparks that flash as electricity jumps between wires.

What is Electricity?
[edit | edit source]Electricity is the physical phenomena associated with the motion of electrons. Typically, electrons are locked to atoms by their attraction with protons that reside in the nucleus or center of atoms. Electrons exhibit a negative charge (−) and are attracted to positively charged (+) matter, such as protons. Special material composed of metallic bonds is conductive to electron motion, due to the fact that electrons can easily move between atoms linked by metallic bonds. Copper, iron, nickel, and gold all make good conductors for the motion of electrons. Electrons can also move through polarized molecules (molecules that have positively and negatively charged poles or sides). This is why electrons can pass through water with dissolved salts, living tissue, and various liquids with dissolved polarizing molecules, and why it is dangerous to touch a charged electric current and why you receive a shock when you do so.

The free flow of electrons is called plasma, and occurs when electrons are stripped from atoms. A good example of plasma is lightning in a thunder storm, which is the free flow of electrons between negatively charged clouds, and the positively charged ground. Electrons move across a wire in a current from the negative toward the positive charged ends of the wire. When electrons move across a wire, they generate an electromagnetic field, such that a compass laid within this invisible field will reorient its needle to this magnetic field. This electromagnetic field was first investigated by Michael Faraday, and has led to an amazing assortment of inventions used in our daily lives, such as electric motors used in electric vehicles. When electrons are in motion they can drop into lower energy states and release electromagnetic radiation, or light. This is what powers light bulbs, computers, and many of the electric devices that we use in our daily lives.
How do you make electricity?
[edit | edit source]How is the flow of electrons generated? How do you make electricity? Well there are four fundamental methods of electric generation.
1. Electromagnetic radiation or light, such as sunlight. When photons strike electrons, they increase their energy states. This was famously demonstrated first by Heinrich Hertz. When electrical sparks are exposed to a beam of UV-light, the wavelength of the light in the spark shifts from longer to shorter wavelengths. This interaction between electromagnetic radiation and electrons is called the photoelectric effect. This is how solar power works, such as solar panels that generate electricity, but is also how living plants generate energy through photosynthesis.
2. Kinetic Energy. The motion of materials can strip off electrons from materials, generating an electric charge. Such demonstrations of this can be seen with the build-up of static electricity in materials which can gain an excess of electrons due to two materials being in contact with each other, with one type of material as an insulator (meaning it prevents the flow of electrons between atoms), and the other type of material as a conductor (meaning it allows the free flow of electrons between atoms). Electrons will build up, on the surface of the conducting material and is discharged as a spark or an electrostatic discharge. Industrial power plants most often utilize this type of electric generation, using motion. Large magnets rotate within closed loops of conducting material (such as copper wire), drawing electrons into the copper wire, which flow out on electric lines to homes and businesses. Large rotating turbines are often powered by hot steam (coal, natural gas, nuclear, or geothermal power plants), the flow of water (hydroelectric dams), or wind (wind turbines) that keep the conducting material rotating and generating electrons.
3. Thermal Energy. Electricity can be generated by a thermogradient, where a heated surface is placed in close association with a cold surface, and two materials with differing electric conducting properties are placed between the thermogradient, allowing the build-up of electrons on one side, generating a current with the opposite side. Thermoelectrically generated electricity is used in electrical generation of wearable devices, which utilizes the thermal gradient of a person’s body heat. It also is used to generate electricity from “waste heat,” that is heat that is generated by the combustion of fuels, such as in a combustion engine or power plant, as a secondary method to boost electrical generation. Such conversion between thermal energy to electrical energy can allow you to charge your cell phone simply by using the heat in a cup of coffee or tea, as demonstrated recently by the work of Ann Makosinski showcased on the Late-Night Show.
4. Chemical Energy. An electrical charge can be built up and stored in a battery. The term battery was first coined by Benjamin Franklin, who took a series of Leyden Jars and lined them in a row connected by metal wires to increase the electric shock he received when he touched the top of a Leyden Jar. With a row of these jars lined up, they resembled a row of cannons, a reference to the military term for a “battery” of cannons. Leyden Jars do not generate electricity on their own, but allows an easy way to store electrons and an electric charge.
Batteries
[edit | edit source]
As the simplest type of battery, a Leyden Jar is a jar wrapped in a conducting metal, filled with a conducting liquid (typically water with dissolved salt), with a nail or metal wire dropped through the lid, making sure that the outer metal does not come in contact with the metal wire or nail in the lid. Using a rod and clothe, electrons can be added to the jar’s lid, by passing a charged rod (after rubbing it with a cloth to build up a static charge), and the electrons will flow into the nail (called an anode, or − end) and into the water (referred to as an electrolyte). Since these electrons cannot pass through the glass jar to the outer surface metal (called the cathode, or + end), they will collect within the jar, until a circuit is made between the lid (anode or − end) and outside of the jar (cathode or + end). If this circuit is made by a person, they will feel a shock. If a wire is attached with a light bulb, the light bulb will light up.
Modern batteries can generate electricity by having two different types of liquid electrolytes separated by a membrane that allows the passage of electrons, but not the molecules in the liquid. Hence, overtime electrons will accumulate in one side (becoming negatively charged), while depleted in the other side (becoming positively charged) of the two chambers of electrolytes. Some batteries, once the electrons have returned to the other side, will be expended, while others will allow a reverse charge to be applied to the battery (a flow of electrons in the opposite direction), which resets the difference in the number of electrons between the two chambers of electrolytes, and hence re-charge the battery. However, over time, the molecules will lose their chemical abilities to donate and receive electrons, and even rechargeable batteries will have a limited life-span. However new technologies are increasing the length of battery life, particularly with molecules that contain the highly reactive element of lithium.
Most often chemical energy generates heat through an exothermic chemical reaction (such as the combustion of gasoline), and heat is then used to generate electricity in one of the ways mentioned previously.
When electrons move along a conducting material in a single direction of flow, this is referred to as a direct current or (DC), which is common in batteries. However, often electrons are passed through an alternator which produces a flow of electrons alternating back and forth along the wire in waves, which is called an alternating current (AC). Typically, electricity in most electric appliances in your home run on alternating current, because it is more efficient in transporting a continuous flow of energy long distances over metal wires. However, most batteries provide electrons through direct current.
Sunlight as an Energy Source for Earth
[edit | edit source]Sunlight is the ultimate original source of most electrical generation for planet Earth. Electric energy can be stored for long periods of time as chemical energy, such as in batteries, but also in ancient fossilized lifeforms which previously used photosynthesis to produce hydrocarbons, which are broken down over long geological periods into natural gas, gasoline, or coal. These “fossil fuels” can combust and generate heat in exothermic reactions to generate electricity through heat and motion.
Theoretical Nature of the Universe’s Energy
[edit | edit source]Scientists have debated the theoretical nature of the universe in regard to the long-term trend of energy available. Lord Kelvin, and the classical laws of thermodynamics view energy as slowly being depleted from the universe due to entropy, and eventually the universe will face a “heat death,” when all the energy has been depleted. Other scientists, discovering the link between matter and energy, such as Albert Einstein, who suggests a balance of energy flow between matter and energy, extending the life of the universe. While more recently, scientists have hypothesized increasing energy far into the future toward a “Big Crunch” or “Big Bounce” where all matter could come back together in the universe, and maybe cycle back to another Big Bang. Such cosmological hypotheses, while of interest, do not yield much support from scientific evidence so far gathered. However, there is evidence for the ongoing rapid expansion of the universe, suggesting that the expanding universe is slowly losing energy overtime, as if the universe is one long extended massive explosion ignited with a Big Bang.
Red-shift
[edit | edit source]When Edwin Hubble studied the visual spectrum of star light at the observatory in Mount Wilson, California, he could calculate the temperature and composition of these far away stars. Now, with the ability to determine distances to these stars by comparing brightness and periodicity, he noticed a strange relationship. The farther away a star or galaxy was from Earth, the more the visual spectrum was shifted toward the red side, such that absorption lines were moved over slightly toward longer wavelength light. In measuring this shift in the spectrum of star light, Hubble graphed the length of this shift versus distance to the star or galaxy observed, and found that a greater shift was observed the farther the distance to the star or galaxy.

This phenomenon became known as the red-shift. Hubble used this graph to calculate what has since been named the Hubble Constant, which is a measure of the expansion of the universe. Hubble first published his estimate of this expansion using the notation of kilometers per second per Mpc (megaparsec). A megaparsec is a million parsecs, or equivalent to 3.26 million light years, or 31×1018 km. It is an extremely long distance. Astronomers argued about his first estimates, and the next hundred years there has been continued debate over the exact value of Hubble’s Constant.
An Earth orbiting satellite was launched in 1990 that bears Edwin Hubble’s name, the Hubble space telescope attempted to address this question. Above the atmosphere of Earth, the Hubble telescope was able to measure the red-shift of distant stars as well as their periodicity in brightness, allowing for a refined measurement of this constant, which was found to be 73.8 ± 2.4 km/s/Mpc. For every megaparsec (about 3.26 million light years) distance, the universe is expanding 73.8 km/sec faster. A star 100 Mpc away from Earth would be expanding at 7,380 km/sec from Earth.
While another telescope, bearing Max Planck’s name, the Planck spacecraft launched by ESA in 2009, has looked at the invisible microwave electromagnetic radiation coming from the universe which also exhibits a red shift, and found a slightly slower expansion of the universe of 67.8 ± 0.77 km/s/Mpc. This measurement is the growing distance between stars.

One way to imagine the universe, is as rising bread dough, with the stars as chocolate chips spread throughout the dough. As the dough rises, or expands, the distance between each of the chocolate chips within the dough increases. This expansion can be faster than the speed of light because nothing is traveling that distance, but the distance itself is expanding between points.
Using the 73.8 km/s/Mpc found with the Hubble telescope, and the discovery of the farthest object observed from Earth (the galaxy GN-z11 in the constellation of Ursa Major), measured at 112,738 Mpc away from Earth (with a redshift of 11.1). The distance between Earth and this distant galaxy GN-z11 is expanding about 28 times faster than the speed of light! In other words, the last time Earth and GN-z11 shared the same space was 12.940 billion years ago, with a distance expanding ever faster away from each other. If we play this universe expansion backward, we find that universe is estimated to be about 13.5 billion years old, and has been expanding outward faster than the speed of light in every direction from Earth. Note that this rate of expansion of the universe expressed within the distance of 1 meter is an expansion of only the width of a single atom, every 31.7 years. Since the formation of the Earth 4.5 billion years ago, the expansion of the universe has added only 1 centimeter per meter. However, over the vast distances of space, this universal expansion is relatively large.

Stephen Hawking wrote in one of his lectures before his death in 2018 “The expansion of the universe was one of the most important intellectual discoveries of the 20th century, or of any century.” Indeed, from the perspective of someone living on Earth, it is as if all the stars in the night sky are racing away from you, like a cosmic children’s game of tag, and you are it. This expanding universe is conclusive evidence of the complete isolation of the solar system in the universe, as well as the extreme precious and precarious nature of planet Earth.
| Previous | Current | Next |
|---|---|---|

