Perspectives of Aquatic Toxicology/Printable version
| This is the print version of Perspectives of Aquatic Toxicology You won't see this message or any elements not part of the book's content when you print or preview this page. |
The current, editable version of this book is available in Wikibooks, the open-content textbooks collection, at
https://en.wikibooks.org/wiki/Perspectives_of_Aquatic_Toxicology
Preface
“It is the supreme art of the teacher to awaken joy in creative expression and knowledge” - Albert Einstein
The Wikibook - Perspectives in Aquatic Toxicology – is primarily written by graduate students of Iowa State University. This Wikibook is the result of the Experimental Course - Aquatic Toxicology (A ECL 444/544X / TOX 444/544X) implemented, and designed by me (the editor) in spring 2019. During the many years of previous studies in my youth, I often felt constrained by the boundaries of textbooks that the teachers were imposing on me. I felt as there was no room to expand the knowledge beyond the colorful hardcovers of a textbook and it’s content. There was no reason for me to be creative, to want more, to ask questions, to seek answers, as it was already predetermined that all I, and thousands of other bright minds, need to know was already in the textbook. Even, there was no need for the teacher. All that was required was the textbook. My homework would be discarded and stowed away in some box, never again to see the sunlight, no matter how creative I tried to be. But, I wanted more...
I created the Aquatic Toxicology course to follow the open pedagogy approach “in which students are active and visible participants in the construction of knowledge” (DeRosa & Robison, 2017, p. 115). This time the students create their own textbook, selecting, and writing their own chapters while transferring the knowledge to each other in the class. There are no hardcovers or boundaries. The book is free and accessible to any student in the world. This collaborative work between the course instructor/editor and the students aimed to present perspectives in Aquatic Toxicology and to establish authors a theoretical foundation for the experience.
While Aquatic Toxicology is a post-World War II discipline, it was practiced for thousands of years. Ancient scholars were conducting research on water pollution oblivious of the fact that such philosophy branch would be dubbed the Aquatic Toxicology many years later. It was Aristotle who invented “aquatic toxicity test” – the foundation methodology behind Aquatic Toxicology designed to assess the potential for damage to an aquatic environment. Around the year 350 BC Aristotle noticed the putrid smell arising from Athens sewage effluent streams and the downstream change in color where the effluent conjoined the pristine stream. He questioned the safety of the Athens drinking water supply and transferred midge fly (chironomids) larvae from pristine stream to the effluent in order to monitor their survival. Some 2,500 years later exposure of model species to effluents is still the basis of many toxicity tests. Later, perhaps what is known to be the first man made aquatic environmental catastrophe occurred in Ancient Rome. The elaborated network of drinking water supply and sewage disposal in large Roman towns were lead pipes that over the course of centuries leached lead into the Tiber River and build-up its deposits in the Portus – an Ancient Rome harbor. It was only recently that scientists discovered that the lead deposits in the sediments beneath Portus around 250-100 AD were many times higher than what was the natural background. Such concentration likely and significantly altered the community of benthic organisms at the harbor. Concomitantly the Roman drinking water had lead concentrations roughly 100 times higher than natural spring water, all while Ancient Romans were unaware of these facts and any associated potential health and environmental issues. History was repeating itself over the course of time and the environmental and health effects of mercury, DDT, dioxins, and other chemicals once thought to be safe, become apparent years after its introduction in the aquatic environment.
Today, Aquatic Toxicology, as a discipline, is getting an increase in global attention due to the explosive growth of the human population, dwindling clean water resources, water pollution, eutrophication, global decline in biodiversity, and increase in the number of newly synthesized chemicals evaluated by the regulatory agencies. Therefore, Aquatic Toxicology is not of interest only to toxicologist but has a much wider audience comprised of ecologists, chemists, risk managers, and environmental scientists, among others.
This Wikibook was supported by The Miller Faculty Development Fund of Iowa State University, specifically The Miller Open Education Mini-Grant. The Miller Fund was made possible by the generosity of F. Wendell Miller, who left his entire estate jointly to Iowa State University and the University of Iowa. The Miller Open Education Mini-Grants aim to support faculty development through the use of new and innovative resources in the classroom. They provide faculty with opportunities to enhance their scholarship of teaching and learning by integrating Open Educational Resources (OER) into their teaching.
This book is not finished and it will be updated with new chapters and new information each time the course is offered. I wish to extend my acknowledgments to Dr. Evrim Baran, the open pedagogy advisor, and to Dana AlZoubi, technical editor of this book.
In Ames, Iowa, on June 30, 2019
Boris Jovanovic, PhD - Editor
Contributors' Biographies
Aquatic Toxicity Tests
Chapter One: Aquatic Toxicity Tests
[edit | edit source]INTRODUCTION
[edit | edit source]Aquatic species are vital to our planet. Phytoplankton, algal plankton, and kelp are major sources of the planet’s oxygen. They absorb and store carbon dioxide, and maintain a hospitable climate. They also play an important role in the global nitrogen cycle and support aquatic animals such as fish, mollusks, sponges, and corals. Aquatic species help maintain the earth’s ecosystem and help preserve its rich biodiversity as well as providing food, medicine, livelihoods, tourism, and recreational opportunities1.
It is therefore essential to protect the planet’s rich and diverse aquatic life, and combat the many threats facing aquatic organisms including climate change, habitat destruction, overfishing, the introduction of invasive species, and chemical pollution2. This chapter will focus on chemical pollution. The risks to aquatic life can be minimized and better managed by understanding how chemicals impact it.
There are more than 140,000 man-made chemicals in the environment3, with the United States alone producing 2000 new chemicals every year4. It is conceivable that aquatic species are exposed to many of these chemicals on an acute (short-term) and chronic (long-term) basis, although there is an absence of data to indicate how many of these chemicals are released into various water bodies. Chemical exposure can affect organisms’ growth, development, fecundity, behavior, and survival, among other biological processes. Hence, it is important to test chemical toxicity before it is released into the environment in order to determine maximum acceptable toxicant concentrations (see section II of this chapter) and to protect species from potential harm.
Toxicity testing is done to identify the degree to which chemicals can damage living organisms in a controlled environment. It has four major objectives:
a. To obtain toxicity and exposure data for various chemicals
b. To aid in estimating and managing risks posed by various chemicals
c. To aid in setting chemical regulations and environmental standards
d. To classify chemicals based on how toxic they are to various species
The dose makes the poison in toxicology. It is possible to determine safe and unsafe doses, or concentrations, for nearly every chemical. For example, the most toxic substance on earth, the bacteria-produced botulinum toxin, can kill humans with a very small dose, but it can be used safely in Botox5.
Risk is a function of toxicity and exposure. A chemical can be very toxic, but it will have zero risk to aquatic organisms if it never enters water bodies (i.e., there is no exposure). The maximum allowable concentration for a chemical in the environment is based on the risk it poses to various species. An acceptable “safe” concentration is usually one that does not harm 95% of the species.
Many questions can be answered by carrying out toxicity tests:
a. At what concentration is a chemical non-toxic to an organism? At what concentration is it toxic?
b. What effects can be observed from short-term and long-term chemical exposure?
c. Which chemicals are the most and least toxic to an organism?
d. Which organisms are the most or least sensitive to a chemical?
e. Are some life stages of an organism more sensitive?
f. Do certain environmental conditions make a chemical more toxic?
g. Is the toxicity of a chemical similar in lab and in the outside environment?
h. What is the effect of a mixture of chemicals?
And more.
Aquatic organisms can be exposed to chemicals when effluents and sewage are released into water bodies. Sometimes chemicals inadvertently enter water through oil spill or runoffs from agricultural fields. Chemicals present in the air can be deposited into water bodies either directly (dry deposition) or through rainfall, snowfall, and fog (wet deposition). Some of the chemicals commonly found in water bodies include detergents, fertilizers, pesticides, pharmaceuticals, food and cosmetic preservatives, chemicals used in kitchenware and plastic, and metals6-8. Aquatic animals such as fish can take up these chemicals via their gills, absorb them through their integument, and/or ingest them. Aquatic plants that have vascular systems can absorb chemicals through their epidermal surface and/or roots. Plants that are not completely submerged in water can take up chemicals in the air through their stomata.
Chemical properties and type of aquatic species determine how chemicals are taken up, distributed, stored, metabolized, and excreted. Hydrophobic (fat-loving) chemicals are more likely to enter a fish’s body, and warm temperatures typically increase the uptake as the fat become more fluid-like. Smaller, uncharged molecules also cross membranes more easily. Hydrophilic (water-loving) chemicals are more likely to be transported by the circulatory system. On the other hand, hydrophobic chemicals are more likely to bind to molecules and accumulate in fat bodies. While chemical storage is protective in the short term (they are not free to move and act), they can be released later and cause toxicity. This usually happens when an organism breaks down fat for greater energy needs, i.e., during illness, starvation, or reproduction.
A species’ metabolic enzymes often modify a chemical in order to detoxify its effects, but this modification can sometimes make a chemical more toxic. Chemicals with many halogen atoms such as chlorine, and fluorine are often difficult to modify. Many aquatic animals eliminate chemicals through their gills or skin. Further details on chemical biotransformation can be found in the Biotransformation of Xenobiotics Chapter of this book.
Chemical exposure can kill or harm aquatic organisms directly through such means as growth reduction, delayed development, decreased fertility, and behavioral changes, or can reduce or eliminate its food supply by killing its prey, or limiting its shelter through habitat destruction. This can lead to increased competition for food and shelter, disrupting the food web, and altering the ecological balance.
BASIC CONCEPTS
[edit | edit source]Units of concentration
[edit | edit source]Concentration is used more commonly than dose in aquatic toxicology. This is because water chemical concentration is easier to measure than the amount of chemical taken up by fish through its gills, integument, and mouth. Concentration of a solution is defined as the ratio of solute to solvent, or the ratio of solute to total solution. This can be either expressed as mass of chemical per unit volume (e.g. mg/mL) or the number of moles of chemicals per liter of solution (e.g. mol/L).
Terms like ppm, ppb and ppt are also often used to describe units of concentration:
a. Parts per million (ppm) corresponds to 1 mg of chemical/L of solution. The amount of chemical is six orders of magnitude lesser than the amount of solution. This is like emptying a large soft drink bottle of a chemical into an Olympic-sized swimming pool.
b. Parts per billion (ppb) corresponds to 1 µg of chemical/L of solution. The amount of chemical is nine orders of magnitude lesser than the amount of solution. This is like putting half a teaspoon of a chemical into an Olympic-sized swimming pool.
c. Parts per trillion (ppm) corresponds to 1 ng of chemical/L of solution. The amount of chemical is twelve orders of magnitude lesser than the amount of solution. This is like putting one-twentieth of a drop of chemical into an Olympic-sized swimming pool.
Concentration and response
[edit | edit source]There is a relationship between the chemical concentration to which an organism has been exposed and the resultant nature and degree of harmful effects. However, it is important to note that the chemical concentration that enters an organism is typically higher than the concentration that causes a toxic effect. This could be due to the organism producing enzymes or molecules that break down or bind to the chemical. This reduces the availability of the chemical to the body, and thus a lower concentration binds to the site of action and exerts a toxic effect.
Assumptions in a concentration-response relationship are as follows:
a. It is a cause-and-effect relationship, i.e., the response occurs due to the organism’s exposure to a chemical.
b. The response is due to a chemical interacting at the site of action.
c. The concentration of a chemical at the site of action is a function of how much chemical the organism was exposed to.
d. Above the threshold concentration (concentration at which a response can be detected), the magnitude of response is proportional to the amount of chemical interacting at the site of action.
e. The response can be measured and reproduced under similar conditions.
The duration of an organism’s chemical exposure is also significant. If a fish is exposed to a low chemical concentration for only one day, it may be unaffected, but if it is exposed to the same concentration for months it may develop cancer, skeletal abnormalities, issues with fertility, etc.
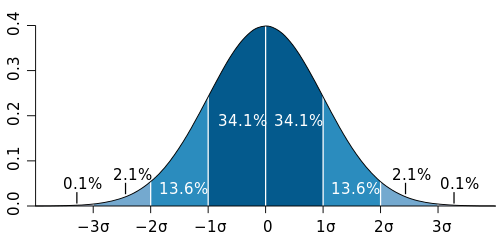
The response to a chemical for any given species usually follows a normal distribution or bell-shaped curve (see Figure 1). This means that some organisms of the same species are very sensitive to a given chemical, some are very resistant, while most are neither very sensitive nor very resistant.
Creating a toxicity curve
[edit | edit source]A toxicity curve or a concentration-response curve is a graph which plots the results obtained from a traditional concentration-response toxicity test. The toxicity test should ideally fulfill the following criteria:
a. There should be five different concentrations of a chemical plus a negative control.
b. The concentrations should be equally spaced.
c. The concentrations should cause a range of effects, from 0% effect to 100% effect.
d. The study should be replicated at least three times.
The toxicity curve generated from such an experimental design should have the following features in most cases (see Figure 2):
a. The x-axis should be the concentration and the y-axis should be the response.
b. The curve should be sigmoidal in shape above the threshold dose.
c. The average cumulative response from the three replicated studies should be plotted with the 95% confidence intervals.
The slope of a generated toxicity curve can indicate several things. A very steep slope indicates that small increases in concentration caused large increases in response; a flat slope indicates that large increases in concentrations caused small increases in response. The curve can also be used to estimate a concentration that causes a particular response (for example, a 10% response). Typically, the concentration which causes a 50% response is calculated, as it has the least variability/noise.

A non-standard toxicity curve is observed for essential chemicals such as water, oxygen, and vitamins. There is an optimal range for these chemicals: a very low concentration causes deficiency and death, and a very high concentration causes toxicity and death (see Figure 3). Another kind of non-standard toxicity curve is seen with chemicals found in such materials as plastics. These chemicals have different modes of action at different concentrations: they can disrupt hormones at low concentrations but not at high concentrations9. Some toxicity curves can be bimodal if males and females have different toxicity thresholds to a chemical.

Measures of toxicity
[edit | edit source]Toxicity is commonly measured in toxicological studies as follows:
Lethal Concentration 50 (LC50): Concentration at which 50% of the organisms in a population are killed following chemical exposure.
Effective Concentration 50 (EC50): Concentration at which 50% of the organisms in a population are affected following chemical exposure. The effects observed could be reduced growth, delayed development, etc.
Inhibitory Concentration 50 (IC50): Concentration at which 50% of organisms in a population are inhibited following chemical exposure. The chemical could have inhibited a specific biological or biochemical function.
Often LC/EC/IC 10 (harm to 10% of population) and LC/EC/IC 90 (harm to 90% of population) are also calculated.
No Observed Effect Concentration (NOEC): Highest chemical concentration that does not cause a toxic effect in the treated population.
Lowest Observed Effect Concentration (LOEC): Lowest chemical concentration that causes a toxic effect in the treated population.
Maximum Acceptable Toxicant Concentration (MATCC): Concentration between NOEC and LOEC, or is a geometric mean of the two. It is calculated for chronic studies only.
TOXIC EFFECTS OF CHEMICALS
[edit | edit source]Chemicals can exert toxic effects on organisms through various mechanisms:
a. Binding: Chemicals can bind to molecules on the surface of cells and disrupt communication between cells. If an organism’s cells do not communicate with each other they will not function normally. Chemicals can also bind to enzymes and prevent them from carrying out essential activities such as digestion and metabolism, as well as bind to DNA and change the amount of proteins produced. A correct number of proteins are needed to build cells, produce hormones, and maintain immunity.
b. Bioaccumulation: This occurs when an organism takes up a chemical faster than it eliminates it. In bioaccumulation the chemical is taken up by contact, respiration, and ingestion. The term bioconcentration is used when the chemical is taken up through contact and respiration only. A chemical accumulation in living tissue can poison tissue, and subsequently, organs.
c. Interaction: Two or more chemicals in an organism can interact with one another. Usually, the combined chemical effect will equal the sum of their individual effects (1 + 2 = 3). This is called an additive effect and can be observed when aspirin and acetaminophen are taken together. They both act in a similar manner and their combined effect is comparable to taking two doses of one drug. Another kind of interaction occurs when the combined effect of two chemicals are greater than the sum of their individual effects (1 + 2 = 5), and can happen if chemical A increases the activity of chemical B. This is called the synergistic or potentiation effect and can be observed when acetaminophen and alcohol are taken together. Both are broken down by the liver and their combined presence taxes the organ, making it more vulnerable to failure. The final kind of interaction occurs when the combined effect of two chemicals is less than the sum of their individual effects (1 + 2 = 1). This occurs if chemical A hinders the activity of chemical B, and is called an antagonistic effect. A classic example of this effect is anti-venom drugs canceling the effects of a snake bite.
The intracellular effects of a chemical can also be described with the help of a toxicity pathway. It is a sequence of events, starting with a chemical entering an organism. A proportion of the chemical reaches the target tissue and interacts with it: for example, by binding to cell receptors on the tissue. This causes a perturbation, or disturbance, in normal cell function. If the cell starts to alter and the organism corrects the change in time, there will not be a problem; however, if the organism does not alter and effect change, the cell will be permanently altered/injured10 (see Figure 4).

Adverse Outcome Pathways (AOPs) encompass toxicity pathways and beyond. That is, they include chemical-molecular interactions and cellular changes, and determine how this leads to changes in organs, organisms, and populations. In Figure 5 an AOP has been drawn for a male fish exposed to a chemical that activates the estrogen receptor. At the cellular stage this could lead to the transcription and production of abnormal proteins. These proteins could cause both ovaries and testis to develop, altering the secondary sex characteristics of the fish and impairing its fertility. The sex ratio could become skewed if many males in a population become feminized and infertile11.

Table 1 shows how toxicity endpoints could manifest in various aquatic organisms. The length of chemical exposure (short-term vs. long-term) often determines which toxicity endpoints should be measured. For example, reproductive problems and tumors are only observed with long-term exposures.
Table 1: Toxicity endpoints for different aquatic species following exposure to chemicals
| SPECIES | TOXICITY ENDPOINTS COMMONLY MEASURED |
| Annelids | Growth, fecundity, bioaccumulation |
| Algae | Growth, biomass, coloration |
| Plants | Growth, length, yield |
| Insects | Mortality, immobility, development, fecundity, emergence, sex ratio |
| Mollusks | Mortality, growth, fecundity, bioconcentration |
| Crustaceans | Mortality, growth, immobility, fecundity |
| Amphibians | Mortality, growth, development, length, histopathology, metamorphosis, reproductive maturity |
| Fish | Mortality, no heartbeat, loss of swimming equilibrium, developmental deformities, length, yolk coagulation, growth, skeletal abnormalities, tumors, reproductive maturation, fecundity, histopathology, egg hatching, behavior, etc. |
TYPES OF TOXICITY TESTS
[edit | edit source]Aquatic toxicity tests can be divided into categories as described below and summarized in Figure 6.

Based on duration of chemical exposure
[edit | edit source]a. Acute: Short-term tests. For fish the tests are 24-96h long; but, for microalgae or bacteria, a 96h test could represent a chronic, life cycle, or multigenerational test. The test is commonly carried out to check chemical lethality.
b. Subchronic: Prolonged acute tests. For fish the tests are typically anywhere between 28 days to 3 months long. The test is usually done to determine if a chemical impacts the growth (body mass) of a species.
c. Chronic: Lasts for at least 10% of the tested species’ lifespan. For invertebrates, 21-day chronic studies are common. The test typically lasts longer than 6 months for fish. It is often conducted to see if a chemical causes reproductive and developmental effects.
d. Life cycle: Lasts through an organism’s entire life cycle. This can be from egg to sexual maturity, or from egg to egg. This test is done to check if a chemical causes developmental or reproductive effects, as with chronic studies.
e. Multigenerational: Carried out on two or more consecutive generations (parents and offspring). It is usually performed to examine if offspring are affected by parental exposure to chemicals.
f. Early-life-stage: Done on embryos or larval stages. Different life stages of an organism can exhibit different sensitivities to a chemical, with early-life stages often more susceptible.
Based on exposure systems
[edit | edit source]a. Static: Organisms are placed in still water containing a chemical (or in control water). The water is not changed during the test. This system is widely used, but for studies longer than 24h they may not accurately represent chemical effects. This is because the concentration of the chemical may change over time and toxic effects may be produced from a build-up of metabolic byproducts released by the organisms.
b. Renewal: Similar to static, with the test conducted in still water. However, in this test the water containing the chemical (or control water) is regularly changed during the test, usually every 24h, ensuring that the chemical concentration remains stable and that organisms are exposed to clean and fresh water daily.
c. Recirculation: Also similar to static, but the water containing the chemical (or control water) is filtered. This ensures that the water quality does not deteriorate over time. However, filters can add uncertainties to the study as the filter media may interact with the test chemical.
d. Flow-through: Water containing the chemical (or control water) constantly flows in and out of the system, maintaining a high quality flow where the influent and effluent never mix. Pumps control the flow of water and dilutors ensure that the right concentration of chemical is delivered. Flow-through systems mimic the natural flow of water, and though expensive, are regularly used for this reason.
Based on toxicity endpoints
[edit | edit source]These tests are done to determine if a chemical could cause one or more toxicity endpoints in a test organism. The endpoints frequently analyzed include mortality, growth, development, reproduction, immobilization, respiration, endocrine effects, and chemical bioaccumulation. Most standardized studies have been developed with these endpoints in mind.
Based on experimental complexity
[edit | edit source]a. Single species: Tests are conducted on a single species in a lab. They are simple and inexpensive to conduct, and constitute the most common type of test. They are often carried out in a flask, beaker, or some other glass container.
b. Microcosm: Tests conducted on two or more species in an artificial and controlled system. They represent a simplified ecosystem. Microcosms should contain less than 1000 liters of water and can be done indoors (e.g. fish tank) or outdoors (e.g. small ponds).
c. Mesocosms: Tests conducted on multiple species placed in experimental water enclosures. Mesocosms represent a complex ecosystem and mimic natural conditions. The volume of water in the system must exceed 1000 liters, and the test is usually done outdoors. More details on this testing method can be found in the Mesocosm Chapter of this book.
d. Macrocosms: Tests conducted in lakes and on whole aquatic ecosystems. They are the most realistic, but they are very difficult and expensive to conduct. Canada has 58 experimental lakes that are designated for macrocosm studies only.
Based on test media
[edit | edit source]a. Water: Water is spiked with a single chemical or a chemical mixture, and aquatic organisms are exposed to it. The vast majority of aquatic toxicity tests are done on water. The toxicity endpoints of these organisms are compared to organisms exposed to control (non-spiked) water.
b. Whole effluent: Samples of effluents are tested by exposing aquatic organisms to them. It is important to assure that as wastewater effluents are discharged into water bodies they will not harm aquatic organisms--as prescribed by the Clean Water Act. Toxicity endpoints are measured and compared to that of organisms exposed to control water.
c. Sediment: Determines if sediments contain concentrated toxic chemicals that will harm organisms. Sediments are the ultimate repository for many chemicals that enter water bodies. Benthic species such as worms, crabs, clams, and lobsters live in or on sediments. Benthic organisms are exposed to contaminated or spiked sediments in sediment toxicity tests, and their toxicity endpoints are compared to organisms that are exposed to control sediments.
It is important to understand that the five different kinds of aquatic tests mentioned above are not independent of one another; this is just one way to classify them. For example, an acute toxicity test can be done on a single species using the static exposure system. The endpoint could be mortality, and the test could be done to check for the presence of toxic contaminants in whole effluents.
Both the Organization for Economic Co-operation and Development (OECD) and the United States Environmental Protection Agency (USEPA) publish guidelines for conducting various kinds of aquatic toxicity tests. These have been summarized in Table 2, along with a link to each test guideline.
Table 2: The different aquatic toxicity test guidelines published by OECD and EPA
| TEST NUMBER AND NAME | DURATION OF
EXPOSURE |
MAJOR ENDPOINTS MEASURED | LINK TO GUIDELINE |
| Test No. 225: Sediment-Water Lumbriculus Toxicity Test Using Spiked Sediment | Subchronic | Growth, fecundity | OECD 225 |
| Test No. 315: Bioaccumulation in Sediment-dwelling Benthic Oligochaetes | Subchronic | Bioaccumulation | OECD 315 |
| Test No. 221: Lemna sp. Growth Inhibition Test | Subchronic | Growth, yield | OECD 221 |
| Test No. 239: Water-Sediment Myriophyllum Spicatum Toxicity Test | Subchronic | Growth, length, yield | OECD 239 |
| Test No. 238: Sediment-Free Myriophyllum Spicatum Toxicity Test | Subchronic | Growth, length, yield | OECD 238 |
| Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test | Chronic | Growth, biomass, coloration | OECD 201 |
| Test No. 218: Sediment-Water Chironomid Toxicity Using Spiked Sediment | Chronic | Development, emergence | OECD 218 |
| Test No. 219: Sediment-Water Chironomid Toxicity Using Spiked Water | Chronic | Development, emergence | OECD 219 |
| Test No. 233: Sediment-Water Chironomid Life-Cycle Toxicity Test Using Spiked Water or Spiked Sediment | Life cycle | Emergence, sex ratio, fecundity, mortality, development | OECD 233 |
| Test No. 235: Chironomus sp., Acute Immobilisation Test | Acute | Immobility | OECD 235 |
| Test No. 242: Potamopyrgus antipodarum Reproduction Test | Subchronic | Mortality, fecundity | OECD 242 |
| Test No. 243: Lymnaea stagnalis Reproduction Test | Subchronic | Mortality, fecundity | OECD 243 |
| 850.1025: Oyster Acute Toxicity Test (Shell Deposition) | Acute | Growth | EPA 1025 |
| 850.1055: Bivalve Acute Toxicity Test (Embryo-Larval) | Acute | Count of embryos and larvae | EPA 1055 |
| 850.1710: Oyster Bioconcentration Factor | Subchronic | Bioconcentration | EPA 1710 |
| Test No. 202: Daphnia sp. Acute Immobilisation Test | Acute | Immobility | OECD 202 |
| Test No. 211: Daphnia magna Reproduction Test | Chronic | Fecundity | OECD 211 |
| 850.1300: Daphnid chronic toxicity test | Chronic | Mortality, growth, fecundity | EPA 1300 |
| 850.1035: Mysid Acute Toxicity Test | Acute | Mortality | EPA 1035 |
| 850.1020: Gammarid Amphipod Acute Toxicity Test | Acute | Mortality | EPA 1020 |
| 850.1045: Penaeid Acute Toxicity Test | Acute | Mortality | EPA 1045 |
| Test No. 231: Amphibian Metamorphosis Assay | Subchronic | Growth, mortality, development, length,
histopathology |
OECD 231 |
| Test No. 241: The Larval Amphibian Growth and Development Assay (LAGDA) | Early-life-stage | Development, metamorphosis, mortality, growth, reproductive maturity | OECD 241 |
| Test No. 203: Fish, Acute Toxicity Test | Acute | Mortality | OECD 203 |
| Test No. 210: Fish, Early-life Stage Toxicity Test | Early-life-stage | Growth, length, hatching, appearance & behavior, mortality | OECD 210 |
| Test No. 212: Fish, Short-term Toxicity Test on Embryo and Sac-Fry Stages | Early-life-stage | Hatching, mortality, behavior, appearance | OECD 212 |
| Test No. 215: Fish, Juvenile Growth Test | Subchronic | Behavior, appearance, growth | OECD 215 |
| Test No. 229: Fish Short Term Reproduction Assay | Subchronic | Fecundity, yolk protein, sex characteristics | OECD 229 |
| Test No. 230: 21-day Fish Assay | Subchronic | Yolk protein, secondary sex characteristics | OECD 230 |
| Test No. 234: Fish Sexual Development Test | Subchronic | Yolk protein, sex ratio | OECD 234 |
| Test No. 236: Fish Embryo Acute Toxicity (FET) Test | Acute | Mortality, yolk coagulation, heartbeat | OECD 236 |
| Test No. 240: Medaka Extended One Generation Reproduction Test (MEOGRT) | Multi-generational | Mortality, growth, development, sex, fecundity, yolk protein | OECD 240 |
| Test No. 305: Bioaccumulation in Fish: Aqueous and Dietary Exposure | Subchronic | Bioaccumulation | OECD 305 |
DESIGNING A TOXICITY EXPERIMENT
[edit | edit source]The results of a toxicity experiment depend largely on how it was designed. A good study design can ensure that the results obtained are valid, applicable, and reproducible. Below are the major criteria used to ensure good aquatic toxicity test design, but it is important to note that not all tests can satisfy all of the outlined criteria due to the specific nature of various toxicity tests.
a. It should be widely accepted by the general scientific community.
b. It should be standardized (i.e., carried out according to defined protocols) and the results must be replicable in different laboratories.
c. It should be easy to perform and economical.
d. The test species selected must be a well-known model organism (see below).
e. The test should cover a range of concentrations, and at least some of these concentrations should be found in the environment.
f. The duration of chemical exposure must be realistic and manageable (for example, some sharks can live for hundreds of years and it is not possible to expose them to a chemical throughout their lifespan).
g. The test should be statistically sound and robust.
h. The data obtained can be used to estimate risk.
i. The test should be sensitive enough to detect and measure the toxic effects under investigation.
j. The test should be able to predict effects to species outside the lab (i.e., in the environment) and also predict potential effects to similar species.
Major standardized aquatic tests were discussed in the above section, with protocols spelled out by the OECD and USEPA. The International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM), which is now an international organization, have also published a few aquatic test protocols and can be found on their websites (https://www.iso.org/home.html and https://www.astm.org/ respectively).
The easy to administer and economical nature of standardized tests enables them to be routinely performed. This precludes macrocosm and mesocosm tests which are very complex and difficult to perform. While all the standardized toxicity tests mentioned in Table 2 were for single species, the USEPA and OECD have published guidelines for indoor microcosms (USEPA 1900) and outdoor microcosms/mesocosms (OECD draft guidance).
The appropriate selection of test species is critical in toxicity testing. While more details on this can be found in the Model Species in Aquatic Toxicology Chapter, most toxicity tests are carried out on model species which have the following characteristics: a. High sensitivity to various chemicals. b. Easily available and abundant.
c. Easy to rear and culture in the lab and allow for various types of toxicity testing.
d. High survival rate in the lab under normal conditions.
e. Extensive and available knowledge of the organism (information on their biology, physiology, genetics, and behavior).
There is no single aquatic species used in tests that can provide answers to all questions or evaluate all chemical impacts on an ecosystem. It is therefore imperative to test several species from different classes: from algae to invertebrates to fish. Also, it is important to test different life stages in a species as every stage has a different sensitivity and a unique response. The environment in which the organism lives should also be taken into consideration (freshwater/marine and warm/cold water).
Under the United States Endangered Species Act (ESA) it is necessary to ensure that chemicals released into the environment do not harm endangered or threatened species. Since the employment of endangered and threatened species in toxicity tests is discouraged, toxicity values obtained from the most sensitive test species are often used to estimate their risk to a chemical. Also, the acceptable risk for these species is often 10-fold lower than that of more abundant species12.
Toxicity tests should not be conducted without first identifying and including one or more available Estimated Environmental Concentrations (EEC) or Actual Environmental Concentration (AEC) of a chemical. EECs are the estimated concentrations of a chemical in an environment and are usually derived through computer modeling or simple predictions. These models were developed with data obtained from laboratory and environmental studies; two such models are available on the USEPA website13. The Pesticide in Water Calculator (PWC) is used to estimate pesticide concentrations in water bodies that result from pesticide applications to land. The KABAM (KOW (based) Aquatic BioAccumulation Model) is used to estimate potential bioaccumulation of hydrophobic carbon-based pesticides in freshwater aquatic food webs. AECs on the other hand, are based solely on empirical data. They can be measured by taking a water sample from a natural water body or a tissue sample from a wild aquatic organism, making it possible with the help of analytical instruments to find the concentration/dose of chemical in the samples. Various methods and instruments are required to extract and analyze different chemicals.
Estimating the risk to a species from an acute exposure to a chemical, particularly a pesticide, constitutes a Tier I study—a simple laboratory study where worse-case estimates are used to calculate the Risk Quotient (RQ). RQ is defined as the exposure concentration divided by the toxicity concentration. The toxicity concentration is the LC50 or EC50 for an acute exposure. The exposure concentration is the peak concentration of the pesticide in water bodies. If the RQ does not exceed 0.5 for aquatic animals (that is, the exposure concentration is half the toxicity concentration) and 1.0 for aquatic plants (that is, the exposure concentration does not exceed the toxicity concentration), the risk is considered acceptable12.
A Tier II study is conducted if the Tier I RQ is exceeded. Here, the species is subchronically or chronically exposed to a pesticide. The toxicity concentration is the NOEC and the exposure concentration is the average pesticide concentration over 21-60 days in water bodies. If the RQ does not exceed 1.0 (for both aquatic animals and plants), the risk is considered acceptable; a Tier III study is carried out if it is exceeded. These investigations are chronic, life cycle, or multigenerational in nature, and can be done in the laboratory or outside (i.e., microcosm studies). Tier IV are mesocosm or macrocosm studies involving multiple species. Both Tiers III and IV usually involve a comparison of the toxicity endpoints of the pesticide-exposed populations to that of the unexposed populations. The endpoints analyzed are biomass, diversity, species richness, etc. Figure 7 summarizes the risk assessment process14. Additional information can be found in the Risk Assessment Chapter.

The design of a study depends largely on the question(s) being asked. Consideration must also be given to the physiochemical properties of the chemical being tested, its mode of action, its pattern of use, the environmental conditions, and the characteristics of the test organism. The following is a brief overview:
a. Study objective/question: An aquatic toxicity study could be done for various reasons. It could be done to test the quality of a sediment or effluent, to register a field-applied pesticide, to understand the long-term impacts of a chemical on a community, or for routine monitoring purposes. The objective determines the type and number of tests needed.
b. Physiochemical properties of a chemical: Prior to toxicity testing it is important to collect information on the chemical’s structural formula, purity, stability in water and light, partition coefficient, vapor pressure, biodegradability, etc. because its property can influence how it moves, persists, and distributes. For example, chemicals which have poor solubility in water are more likely to accumulate in aquatic organisms or bind to sediment. However, in a lab they may instead bind to the plastic or glass container housing the test organism. Therefore, special steps need to be taken to ensure that the fate of a lab chemical mimics the fate of a chemical in the environment. Similarly, it is not worthwhile to conduct long-term toxicity studies in the lab for chemicals which degrade rapidly in the environment.
c. Mode of a chemical action: Most chemicals found in water bodies have a specific mode of action, i.e., they bind to a specific target site and produce specific downstream effects. Knowledge of a chemical’s mode of action will help decide which endpoints are the most important to measure during and after a toxicity test.
d. Pattern of chemical use (or discharge): A species can be exposed to a chemical only if there is an overlap in space and time. This means that the species must be present at a location where the chemical is found and present at a time the chemical is present in the environment. Some chemicals are only intermittently used and found in the environment. Conducting life cycle or multigenerational studies for such chemicals will not provide much usable information.
e. Environmental conditions: The environment can affect the toxicity and availability of a chemical. High temperatures can more effectively dissolve chemicals, and this might increase the amount of chemical an organism is exposed to. They can also break down chemicals, which can decrease or increase a chemical’s toxicity. Other factors can influence toxicity such as percentage of dissolved oxygen, salinity, nutrient level, moisture level, and microbial community. This makes it important to mimic the natural conditions of a test organism in the lab.
f. Characteristics of the test organism: If the objective of the study is to assess sediment toxicity, then organisms that dwell in or near the bottom of a water body should be tested rather than organisms that dwell near the top, unless a major disturbance of sediment is expected (e.g. dredging). It is necessary to choose species that can coexist together if the objective is to study how a community of aquatic species is harmed by a given chemical.
While specific guidelines for various aquatic toxicity tests were linked in Table 2, the following are general guidelines that apply to all tests:
a. Laboratory toxicity tests should be replicated at least thrice under similar conditions to ensure results are reproducible. Mesocosm tests should be replicated at least twice, while there are no requirements for macrocosm tests (due to their complexity and scale).
b. All toxicity tests should have a negative control. The control should have the same conditions and constituents as the treatment group, minus the chemical that is being tested. If the chemical is dissolved in a solvent prior to its introduction into the water, then the negative control should also contain the solvent. This is done to remove any potential effects of the solvent (though solvents should be tested before the study to ensure they are non-toxic).
c. While most toxicity tests are not required to have a positive control, it is encouraged. A positive control is a substance that is known to produce a defined toxic effect in the test organism. It is used to determine if the health and sensitivity of the test organisms have changed over the course of the study. Also, it can help validate data across different labs and help assess reproducibility of the results.
d. No less than three organisms must be treated for every concentration that is used (including control). However, the minimum sample size often depends on the study type and objective. If too few organisms are treated, it is possible to miss significant differences that might exist between treatments and controls. If too many organisms are treated (more than what is necessary), it would cause ethical issues and lead to wasted time and resources. Therefore, a power analysis is often carried out to find the minimum number of organisms needing treatment to study a particular effect.
e. All organisms used in a test must be homogenous (unless specifically instructed otherwise in the test guidelines). They should be of similar age, life stage, body mass, size, etc. They should all be healthy (sick organisms might be more sensitive to the effects of a chemical) and must have followed similar growth patterns prior to chemical exposure.
f. Randomization of controls and treatments should be done to account for non-chemical effects. For example, if all control organisms are placed on the top shelf where the light source is the brightest, they may grow differently than treatment organisms placed on the bottom shelf due to dissimilar light intensity. The difference could be mistakenly attributed to the chemical in the absence of random placement.
g. Conditions such as temperature, light, oxygen concentration, and hardness of water should be maintained throughout the test environmental to avoid impacting a chemical’s toxicity and/or availability (i.e., exposure).
h. The concentration of a chemical must be measured and maintained throughout the test. Ideally, the chemical concentration must be analyzed in the water, food, sediment, and in test organism tissues: however, most test guidelines only require that chemical concentration be measured in water.
CONCLUSIONS
[edit | edit source]While laboratory toxicity tests greatly help in understanding a chemical’s effects, the observed effects will not necessarily be the same in the natural environment. This is due to several laboratory test limitations: a. In nature, there are seasonal fluctuations, temperature changes, diverse microbial communities, etc. that are not captured in lab studies and which can greatly impact results.
b. Organisms in nature are exposed to multiple chemicals and stressors simultaneously. These chemicals and stressors can interact and produce unexpected results. The majority of lab toxicity tests are conducted with only one chemical and under conditions that are favorable to the test organism.
c. In nature, organisms may not be continually exposed to chemicals. In the lab however, long-term studies are carried out where organisms are continuously exposed to the chemical throughout their life cycle. Also, the concentration and availability of the chemical may vary in the environment while they remain constant in the lab.
d. Most lab studies involve spiking the chemical in water and exposing organisms to it. In nature, organisms are also exposed to chemicals through food and sediment.
e. Different physical habitats of ecosystems as well as the genetic structure of populations can also influence toxicity. Most lab studies are carried out on single species, ignoring species-environment, and chemical-environment interactions.
f. Lab studies are done on healthy organisms that are very similar to one another (due to frequent inbreeding). Organisms in nature are more genetically and physiologically diverse.
g. The results from surrogate species tested in the lab are extrapolated to other species since it is not possible to test the thousands of aquatic species present in nature. This adds considerable uncertainty to the results.
The first known aquatic toxicity test was conducted by Aristotle in the 4th century BC, when he exposed midge fly larvae to Athens’ effluent streams to monitor their survival and behavior. The field has progressed considerably since then, especially in the last century. Since 1899, many environmental protection laws have been introduced around the globe, and several of these laws require the regulation of chemicals prior to its registration and introduction into the environment. This has led to the development and standardization of toxicity tests. The aquatic toxicity test guidelines that are currently being used were developed within the last 27 years. However, these guidelines are not set in stone. A greater understanding of the world and man-made chemicals has led to the revision of old guidelines and the addition of new ones. In addition, separate test guidelines have been written in recent years to accommodate new chemicals with unique properties.
To better comply with increasingly stringent regulatory requirements and chemical testing while raising ethical standards, organizations such as the European Center for the Validation of Alternative Methods (ECVAM), AltTox, National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs), Center for Alternatives to Animal Testing (CAAT), etc., are focused on finding alternatives to testing chemical toxicity in animals.
A seminal report was released in 2007 by the National Academy of Sciences titled Toxicity Testing in the 21st Century: A Vision and a Strategy15. This report recommended that toxicologists move away from using vertebrates in toxicity tests and move toward the use of non-vertebrates and cell lines. Today it is possible to recreate organs on a chip and test chemicals on it16. The report also suggested using computer and mathematical models (examples include Quantitative Structure Activity Relationships, Read-Across17, Species Sensitivity Distribution18, Physiologically Based Pharmacokinetic modeling19) and knowledge-based pathways (examples include Toxicity Pathways and Adverse Outcome Pathways) to predict the toxicity of chemicals and to extrapolate results from cell lines to whole organisms and ecosystems. These technologies are yet to be implemented on a large scale, and it will probably take several decades for this to be realized.
REFERENCES
[edit | edit source]Main reference used
Rand, GM. 2008. Fish Toxicity Studies. In Di Giulio RT, Hinton DE, The Toxicology of Fishes, 1st ed. CRC Press, Taylor & Francis Group, Boca Raton, FL, United States of America, pp 659-681.
Specific in-text references
1. International Union for Conservation of Nature. Our Work-Marine. Switzerland. [cited 2019 May 5]. Available from: https://www.iucn.org/theme/species/our-work/marine
2. Reid, G. McG., Contreras MacBeath, T. and Csatadi, K. 2013. Global challenges in freshwater fish conservation related to public aquariums and the aquarium industry. International Zoo Yearbook 47(1): 6-45. Available from: https://zslpublications.onlinelibrary.wiley.com/doi/abs/10.1111/izy.12020
3. Hartung, T. 2009. Pathway Based Approaches: A European Perspective. Toxicity Pathway-Based Assessment: Preparing for Paradigm Change, a Symposium of the Standing Committee on Risk Analysis Issues and Reviews— May 11–13, 2009. National Research Council, Washington, D.C. Available from: https://www.nap.edu/read/12913/chapter/2#3
4. The American Oil Chemists’ Society. TSCA and the regulation of renewable chemicals. Urbana, IL. [cited 2019 May 5] https://www.aocs.org/stay-informed/inform-magazine/featured-articles/tsca-and-the-regulation-of-renewable-chemicals-july-august-2013
5. Nigam PK, Nigam A. 2010. Botulinum toxin. Indian J Dermatol. 55(1):8–14. doi:10.4103/0019-5154.60343. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2856357/
6. Natural Resources Defense Council. The Most Common Types of Water Contamination. New York. [cited 2019 May 5] Available from: https://www.nrdc.org/stories/water-pollution-everything-you-need-know#common
7. Ferrey M. 2013. Pharmaceuticals and Endocrine Active Chemicals in Minnesota Lakes. Technical report. Minnesota Pollution Control Agency, Saint Paul, MN. Available from: https://www.pca.state.mn.us/sites/default/files/tdr-g1-16.pdf
8. Ferrey M, Streets S, Lueck A. Pharmaceuticals and Personal Care Products in Minnesota’s Rivers and Streams: 2010. Technical report. Minnesota Pollution Control Agency, Saint Paul, MN. Available from: https://www.pca.state.mn.us/sites/default/files/tdr-g1-17.pdf
9. Warner GR, Flaws JA. 2018. Bisphenol A and Phthalates: How Environmental Chemicals Are Reshaping Toxicology. Toxicological Sciences. 166(2): 246--249. https://doi.org/10.1093/toxsci/kfy232. Available from: https://academic.oup.com/toxsci/article/166/2/246/5212891
10. National Institutes for Health, US National Library of Medicine. Cell Damage and Tissue Repair. [cited 2019 May 5] Available from: https://toxtutor.nlm.nih.gov/14-002.html
11. Browne P, Noyes PD, Casey WM, Dix DJ. 2017. Application of Adverse Outcome Pathways to U.S. EPA’s Endocrine Disruptor Screening Program. Environmental Health Perspectives. 125(9) CID: 096001. Available from: https://ehp.niehs.nih.gov/doi/10.1289/EHP1304
12. US Environmental Protection Agency, Washington, DC. Technical Overview of Ecological Risk Assessment: Risk Characterization. [cited 2019 May 5] Available from: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/technical-overview-ecological-risk-assessment-risk
13. US Environmental Protection Agency, Washington, DC. Models for Pesticide Risk Assessment. [cited 2019 May 5] Available from: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/models-pesticide-risk-assessment
14. US Environmental Protection Agency, Washington, DC. Exposure Assessment Tools by Tiers and Types - Screening-Level and Refined [cited 2019 May 5] Available from: https://www.epa.gov/expobox/exposure-assessment-tools-tiers-and-types-screening-level-and-refined
15. National Research Council of the National Academies. 2007. Toxicity Testing in the 21st Century. National Academies Press. Available from: https://www.nap.edu/read/11970/chapter/1
16. Kwon D. 2017. Organs on Chips. [cited 2019 May 5] Available from: https://www.the-scientist.com/news-opinion/organs-on-chips-31020
17. AltTox.org Non-Test Approaches: (Q)SARS, READ-ACROSS [cited 2019 May 5] Available from: http://alttox.org/mapp/emerging-technologies/non-test-approaches-qsars-read-across/
18. US Environmental Protection Agency Washington, DC. Species Sensitivity Distributions. [cited 2019 May 5] Available from: https://www.epa.gov/ceam/species-sensitivity-distributions
19. US Environmental Protection Agency, Washington, DC. Physiologically-Based Pharmacokinetic (PBPK) Models [cited 2019 May 5] Available from: https://www.epa.gov/sites/production/files/2018-02/documents/pbpk_factsheet_feb2018_0.pdf
GLOSSARY
[edit | edit source]Actual Environmental Concentration (AEC): The measured concentration of a chemical in the environment.
Acute exposure: Short-term exposure to a chemical.
Acute toxicity test: Short-term toxicity tests that are usually 24-96h long.
Additive effect: Combined effect of two or more chemicals is equal to the sum of their individual effects.
Adverse Outcome Pathway (AOP): A structural representation of a sequence of biological events that leads to adverse effects, from the cellular level to the population level.
AltTox: A website dedicated to advancing non-animal methods of toxicity testing.
American Society for Testing and Materials (ASTM) International: A nonprofit international organization that develops and publishes procedures for testing chemicals and materials.
Analytical instruments: Instruments that can measure the physical and chemical properties of compounds.
Annelids: Ringed or segmented worms. Examples include earthworms, leeches and ragworms.
Antagonistic effect: Combined effect of two or more chemicals is less than the sum of their individual effects.
Benthic species: Organisms that live in the lowest level of water body. They live in, on, or near sediments. Examples include worms, crabs, clams and lobsters.
Bimodal distribution: The observation of two peaks when the data is graphically represented, unlike a normal distribution where only one peak is observed.
Bioaccumulation: Uptake and retention of chemicals through contact (integument), respiration (gills), and ingestion (mouth).
Bioconcentration: Uptake and retention of chemicals through contact (integument) and respiration (gills).
Biodegradability: Breakdown of chemicals by microorganisms, such as bacteria and fungi.
Biomass: The total mass of organisms in a given area or volume.
Cell lines: Cells that are isolated from organisms and grown in the lab under controlled conditions.
Cell perturbation: Alteration in the functioning of the cell.
Center for Alternatives to Animal Testing (CAAT): An academic center at Johns Hopkins University that is working to find new methods to replace, reduce, and refine animal testing.
Chemical purity: The degree to which a substance is undiluted or unmixed with extraneous material. Typically expressed as a percentage (%).
Chemical stability: Tendency of a chemical to resist change or decomposition due to physical, chemical, or biological factors.
Chronic exposure: Long-term exposure to a chemical.
Chronic toxicity test: Long-term toxicity tests that last for at least 10% of the life span of the tested species.
Clean Water Act (CWA): The United States Act that prohibits discharge of toxic pollutants in toxic amounts in water bodies.
Concentration of solution: The ratio of solute to solvent, or the ratio of solute to total solution.
Concentration-Response Curve: Graphical representation of a population’s response to a range of chemical concentrations.
Confidence interval 95%: A range of values within which the true value lies, with 95% certainty.
Crustaceans: Invertebrate animals with external skeleton and a segmented body. Examples include crabs, lobsters, shrimps, and prawns.
Dose: The amount of chemical that enters an organism.
Dry deposition: The direct deposition or settling of chemicals present in the air.
Early-life-stage toxicity test: Toxicity tests that are done on embryos or on larval (juvenile) stages of a species.
Ecological balance: The equilibrium between, and harmonious coexistence of, organisms and their environment.
Ecosystem: A biological community of interacting organisms and their physical environment.
Effective Concentration 50 (EC50): Concentration at which 50% of the organisms in a population are affected following chemical exposure.
Effluent: Liquid waste or sewage discharged into a water body.
Emergence: The appearance of adult insect from its pupal case (i.e., its chrysalis or cocoon).
Empirical data: Evidence gathered from observation or experimentation.
Endangered Species Act (ESA): The United States Act that requires federal agencies to ensure that their actions do not jeopardize the existence of any threatened or endangered species.
Epidermal surface: Tissue that covers the outer surface of plants.
Estimated Environmental Concentration (EEC): The estimated (non-measured) concentration of a chemical in the environment.
European Centre for the Validation of Alternative Methods (ECVAM): An European Union Reference Laboratory that is studying alternatives to animal testing.
Exposure (or exposure concentration): The concentration of chemical an organism encounters in the environment.
Extrapolate: A method to estimate new (unknown) values from old (known) values.
Filter media: Anything placed in a filter that changes the quality of water flowing through it.
Flow-through system: A setup in which organisms are held in continuously flowing water for the duration of the study.
Food web: A system of interdependent food chains. A food chain is a hierarchical series that shows how various organisms obtain food.
Geometric mean: The geometric mean of two numbers is the square root of the product (multiplication) of the two numbers.
Habitat: The natural home or environment of an animal, plant, or other organism.
Halogens: A group of reactive nonmetallic elements that include fluorine, chlorine, bromine, iodine, and astatine.
Histopathology: Microscopic examination of tissues in order to study damage.
Hydrophilic chemicals: Chemicals that easily dissolve in water and typically contain many hydrogen and oxygen atoms.
Hydrophobic chemicals: Chemicals that do not dissolve in water and typically contain many carbon atoms.
Inhibitory Concentration 50 (IC50): Concentration at which 50% of the organisms in a population are inhibited following chemical exposure.
Influent: Something that flows into a system.
International Organization for Standardization (ISO): An independent international organization that sets standards for many materials, products, processes, and services.
Kelp: A type of large brown seaweed.
KOW (Based) Aquatic Bioaccumulation Model (KABAM): A model that can estimate potential bioaccumulation of hydrophobic carbon-based pesticides in freshwater aquatic food webs.
Lethal Concentration 50 (LC50): Concentration at which 50% of the organisms in a population are killed following chemical exposure.
Life cycle toxicity test: Toxicity tests that last throughout the life cycle of an organism.
Lowest Observed Effect Concentration (LOEC): The lowest concentration of chemical that causes a toxic effect in the treated population.
Macrocosm: Toxicity tests that are conducted in lakes and on whole aquatic ecosystems.
Maximum Acceptable Toxicant Concentration (MATC): The concentration between NOEC and LOEC, or a geometric mean of the two.
Mesocosm: Toxicity tests that are conducted on multiple species placed in experimental water enclosures that mimic natural conditions. The system contains more than 1000 liters of water.
Metabolic byproducts (or metabolic waste): The left-over products of metabolism.
Metabolic enzymes: Enzymes that carry out a variety of critical cellular functions in organisms.
Metabolism: The chemical processes that occur within a living organism in order to maintain life.
Metamorphosis: The process of transformation of an organism from an immature form to an adult form.
Microcosm: Toxicity tests that are conducted on two or more species in an artificial and controlled system within a laboratory or outdoors. The system contains less than 1000 liters of water.
Model organism: A species that has been widely studied, is easy to maintain and breed in a laboratory, and has several other characteristics that make it ideal for experimental use.
Mode of action: The mechanism by which a substance produces an effect in living organisms or biochemical systems.
Moles: The quantity of a substance that has the same number of particles found in 12 grams of carbon-12 (6.02x1023 particles).
Mollusks: Invertebrate animals that have a soft unsegmented body. Examples include snails, slugs, mussels, and octopuses.
Multigenerational toxicity tests: Toxicity tests that are carried out on two or more consecutive generations (parents and offspring).
National Centre for the Replacement, Refinement & Reduction of Animals in Research (NC3Rs): A UK-based scientific organization dedicated to finding alternatives to animals in research and testing.
Negative control: A group that has the same conditions and constituents as the treatment group, minus the chemical that is being tested.
No Observed Effect Concentration (NOEC): The highest chemical concentration that does not cause a toxic effect in the treated population.
Normal distribution: The distribution of many random values as a symmetrical bell-shaped graph.
Organization for Economic Co-operation and Development: An intergovernmental economic organization that considers the environmental implications of economic and social development.
Partition coefficient: The ratio of concentrations of a chemical in a mixture of two immiscible (unmixable) liquid phases at equilibrium.
Parts per billion: Corresponds to 1 µg of chemical/L of solution. The amount of chemical is nine orders of magnitude lesser than the amount of solution.
Parts per million: Corresponds to 1 mg of chemical/L of solution. The amount of chemical is six orders of magnitude lesser than the amount of solution.
Parts per trillion: Corresponds to 1 ng of chemical/L of solution. The amount of chemical is twelve orders of magnitude lesser than the amount of solution.
Peak concentration in water: The maximum concentration of chemical estimated or measured in water.
Pesticide: A substance used to destroy pests. The pests can be insects, weeds, fungi, rodents, etc.
Pesticide in Water Calculator (PWC): A model that estimates pesticide concentrations in water bodies following pesticide applications to land.
Physiochemical properties: The properties of a chemical observed from its interaction with the physical environment.
Physiologically based pharmacokinetic (PBPK) modeling: A mathematical modelling technique that predicts the absorption, distribution, metabolism, and excretion of substances in living organisms.
Plankton: A diverse collection of small organisms that live in large bodies of water and are unable to swim against a current. Examples include bacteria, algae, protozoa, and archaea.
Positive control: A group that receives a substance that is known to produce a defined toxic effect.
Potentiation effect: Combined effect of two or more chemicals is greater than the sum of their individual effects. Usually one of the chemicals produces no effect on its own.
Power analysis: A statistical analysis that helps determine the sample size required to detect an effect of a given size with a given degree of confidence.
Quantitative structure–activity relationship (QSAR) model: A model that predicts the biological activities of untested chemicals using their structures.
Randomization: The process of making something random to minimize bias.
Read-Across: A model that predicts the toxicity of untested chemicals using the toxicity information of similar but tested chemicals.
Recirculation system: A setup in which organisms are held in water that is continuously run through a filter for the duration of the study.
Renewal system: A setup in which organisms are held in still water that is regularly changed for the duration of the study.
Resistance: The natural ability of some organisms to withstand the effects of a compound.
Risk: It is the likelihood of a hazard (e.g. a toxic chemical) causing harm.
Risk Quotient: The ratio of chemical exposure to chemical effects.
Sample size: The number of organisms in an experimental sample or group.
Sediment toxicity testing: Toxicity tests where aquatic organisms are exposed to sediments to determine if the chemicals in the sediments will harm them.
Sensitivity: An organism’s susceptibility to the effects of a compound.
Sigmoidal curve: A “S” shaped curve.
Site of action: The location where a compound binds to exert its effects. This is usually a cell receptor, an ion channel or an enzyme.
Slope of curve: The steepness of a curve.
Solvent: A liquid in which a compound is dissolved to form a solution.
Species diversity: The number and distribution of species in the environment.
Species richness: The number of species in the environment.
Species Sensitivity Distribution: A mathematical model that describes the variation in chemical sensitivity between species.
Spiking: The addition of a compound to a solution.
Standard deviation: A quantity that indicates how measurements for a group are spread out from the average or expected value.
Standardization: The process of making something conform to a standard i.e. done according to specific guidelines.
Static system: A setup in which organisms are held in static water that in unchanged for the duration of the study.
Stomata: Minute pores in the epidermis of the leaf or stem of a plant which allows for exchange of gases.
Stressors: Factors that stress organisms. Examples include food shortage, parasites, predators, and changing environmental conditions.
Subchronic toxicity test: Prolonged acute toxicity tests that are typically between 28 days to 3 months long in fish.
Surrogate species: A species that is used to represent multiple species or aspects of the environment.
Swimming equilibrium: The ability that allows fish to maintain an upright position within the water column.
Synergistic effect: Combined effect of two or more chemicals is greater than the sum of their individual effects.
Threshold concentration: The lowest concentration of a chemical that elicits a response.
Tiered Testing: A toxicity testing method where lower tiered studies (representing simple worse-case scenarios) are followed by higher tiered studies (representing complex realistic scenarios).
Toxic effects: The adverse effects produced by exposure to a substance.
Toxicity: The degree to which a substance can harm living organisms.
Toxicity curve: A graph in which chemical concentration (or dose) is plotted against an organism response.
Toxicity endpoint: The measurements and observations taken during or after a toxicity study.
Toxicity Pathway: A sequence of intracellular events that, if disturbed, can cause an adverse outcome at the cellular level.
Toxicity test: A test conducted to find the degree to which a substance can harm living organisms.
Toxicity thresholds: The lowest concentration of a chemical that causes toxicity in organisms.
Uncertainty: Undetermined factors that can produce unreliable results.
United States Environmental Protection Agency (USEPA): A federal agency in the United States that is responsible for registering and regulating chemicals, including pesticides.
Validate: Check or prove the accuracy of (something).
Vapor pressure: A quantity which informs the tendency of a chemical to evaporate.
Vascular system: The presence of xylem and phloem tissues in plants that are responsible for transporting water, nutrients, and food.
Wet deposition: The deposition or settling of chemicals in the air through rainfall, snowfall, and fog.
Whole effluent toxicity testing: Toxicity tests where aquatic organisms are exposed to wastewater effluents to determine if chemicals in the effluents will harm them.
Chapter Two: Bio-transformations of Xenobiotics
Chapter 2: Biotransformations of Xenobiotics
[edit | edit source]Introduction
[edit | edit source]Fish and other aquatic organisms are exposed life-long to the combined effluents of human sources, erosion runoff, and natural excretions from plants and animals. Exposure of aquatic organisms to the chemical mixture is very different from that of terrestrial species like humans. For example, while both humans and aquatic organisms might be exposed to the water-soluble herbicide atrazine, in humans the exposure would most likely be through ingestion of contaminated food or drinking water. Fish would be exposed through their skin and gills. Ingested atrazine first travels to the liver whereas atrazine taken up through gills go directly to the bloodstream. In this way, the environment of terrestrial and aquatic organisms plays a significant role in exposure to various environmental chemicals.
All organisms have defenses to help them deal with and survive potentially harmful chemicals originating from outside the body known as xenobiotics. A large group of these defenses take the form of enzymes that transform a xenobiotic into a different molecule, ideally one that will no longer pose a threat to the host organism. This process is called biotransformation (Figure 1). Biotransformation is the method for metabolic detoxification of xenobiotics. Aquatic organisms have evolved an array of methods to perform biotransformation when they encounter a potent mix of chemicals dissolved in their aquatic environment.

ADME: Absorption, Distribution, Metabolism and Excretion
[edit | edit source]Absorption, Distribution, Metabolism, and Excretion (ADME) are the four steps used to describe the ways a toxicant will interact with an organism to allow or disallow it from inducing a toxic outcome. ADME describes where, when, and how much of a potential toxicant is present (the toxicokinetics), but it does not describe how the toxicant causes harm (the toxicodynamics). Many of the principles of ADME are very similar between related terrestrial and aquatic organisms; however, absorption can be very different due to varying potential exposures when a toxicant crosses an aqueous medium. This differential absorption--based on the physical properties of the xenobiotic compounds--determines which chemicals will be engaged in the third part of the acronym: metabolism.
One physical property that largely influences how a potential toxicant will interact with a body is its propensity to associate with water or lipids. A measure that describes this physical property is the partition coefficient (P) which is often reported as log P. Log P is sometimes written as log Kow where P = Kow = the octanol/water partition coefficient as the measurement is taken while performing a liquid/liquid separation with octanol and water. Higher values of log P mean that a given molecule will spend more time associated with non-polar conditions such as octanol over a highly polar aqueous environment. This has major implications for fish and other aquatic organisms that have fat rich tissues separated from the water-based environment by complex membranes having both non-polar and polar characteristics.
Physical properties of gills: The gills of fish are highly specialized oxygen and ion exchange tissues that include high surface area lamella and extensive vasculature. Even with these specializations, some of the properties of fish gills (e.g. thin membrane size and partial permeability to water, gases, and solutes) allow general comparison to membranes of other taxa such as insect gills that do not have pulmonary vasculature. The gill cells in direct contact with the environment are epithelial cells of various kinds, including lamellar cells that facilitate oxygen exchange and chloride cells that are essential for ion balance (Evans, 1987). Fluids near a solid surface create a slow-moving layer called a boundary layer. When a xenobiotic interacts with the boundary layer of water around a gill, it is called an aqueous diffusion layer. The xenobiotic must cross this layer via diffusion rather than being carried by the flow turbulence due to the relative stillness of this boundary layer (Figure 2). Erickson, et al. (1990) explored how potential xenobiotics cross the aqueous diffusion layer and the cell membrane of the gills in rainbow trout. They found that chemicals of high lipophilicity (log KOW > 3) had low uptake due to an inability to readily diffuse across the aqueous diffusion layer. Chemicals of particularly low lipophilicity (log KOW less than 1) also had lower uptake because it is more difficult to cross the cell membrane which contains lipids. Uptake of chemicals peaked in a Goldilocks zone of moderate lipophilicity (log KOW between 1 and 3), as they could cross both barriers at higher rates. It is likely that other taxa of organisms with gills or permeable skin would have similar chemical uptake profiles in relation to the compound’s log KOW.

The excretion of xenobiotics in aquatic life and land dwelling counterparts is very different. Terrestrial animals typically have some mechanism to withdraw excess water from fecal material to reduce water lost during defecation. Nitrogen waste in the urine depends on the common availability of water to a species. Bursell (1967) describes several different strategies for nitrogen waste removal in insects. Terrestrial insects that reside in dry climates such as the migratory locust (Locusta migratoria), pack their nitrogen waste in the form of insoluble crystals of uric acid and excrete a mostly dry waste. Insects that spend part of their life history in water such as Aeshna cyanea larva, a species of the hawker dragonfly, are more similar to fish in that they produce the potentially toxic nitrogen product, ammonia, but allow it to diffuse into the surrounding water before it builds to harmful levels. Humans take a middle route. We have no sink that allows ammonia to simply diffuse away when it occurs, and we require a relatively high water intake to partially eliminate the need to spend energy packing nitrogen into uric acid. Instead, humans and many other species make urea: a compound with lower toxicity and solubility in-between ammonia and uric acid (OpenStax, 2013). The general excretion strategy used by aquatic organisms is to make use of the surrounding environment by creating a sufficiently water-soluble molecule that will diffuse away from the body.
Biotransformations, Phase I
[edit | edit source]The major metabolic pathways responsible for biotransformation of potential toxicants are called phase I and phase II, although they do not necessarily occur in that order or in sequence at all. Phase I bio-transformations typically employ water or oxygen as an enzymatic factor in reactions such as oxidation, hydrolysis, and reductions. Phase II bio-transformations often form covalent bonds to existing biomolecules in the body. Bio-transformations typically lead to detoxification of a xenobiotic, most commonly by making it more readily excretable. Once a chemical is excreted from the body it is much less likely to cause harm. Changes to the chemical structure can also make a xenobiotic less toxic, even if it is retained in the body.
Oxidation: Many phase I bio-transformations are performed by mixed-function oxidases (MFOs) which are common in the liver as well as in other tissues. A major family of MFOs is called cytochrome P450 (Klaassen, 2013). Cytochrome P450 enzymes perform oxidations (the number in the name refers to the wavelength of light absorbed by the enzyme and helps in their characterization). Many different cytochrome P450’s have been discovered and have a wide range of specificity and substrate compatibility (Figure 3). Stegeman et al. (1991) explored how several carcinogenic toxicants are metabolized by different Cytochrome P-450’s in fish. Distribution of these enzymes were spread out over the locations, liver, kidney, and gills in Scup fish. Despite subtle differences in the specific enzyme used, cytochrome P-450’s are highly conserved. Moktali et al. (2012) trace the evolution of these detoxification enzymes and show that it is more ancient than any terrestrial lineage.

Reductions: Xenobiotics of an elevated oxidation state (certain metals and functional groups such as carbonyls, disulfides, quinones, azo, and nitro groups) can be reduced directly or enzymatically using reducing agents often referred to in consumer foods as “anti-oxidants.” These reducing agents or cofactors include glutathione, FAD (Figure 4), FMN, and NAD(P) (Klaassen, 2013). Many of these cofactors are modifications of basic essential molecules: glutathione is a modified polypeptide and NAD(P) are modifications of dinucleotides. Some enzymes considered oxidases can perform reductions: cytochromes P450 can sometimes use a xenobiotic as the oxidizing cofactor which will effectively perform a reduction on the xenobiotic to oxidize something else. Reductases can vary in specificity. Nitrate reductases are important for the nitrogen cycle and allow plants to utilize nitrate fertilizers. Timmermans, et al. (1994) investigated the effects of available iron on nitrate reductases in phytoplankton and found that the ability to produce a functional enzyme requires the presence of dissolved iron in the environment.

Hydrolysis: Hydrolysis is the process of adding water across a chemical bond. The most generic example is breaking a C-O bond, and making a new C-O bond and an O-H bond (Figure 5). Other bonds can be hydrolyzed as well. The defining characteristic is the consumption of water to split a bond, as shown in blue in Figure D. The process leads to a net zero change in oxidation state: it is neither an oxidation nor reduction reaction. Some compounds undergo hydrolysis spontaneously in water or in plasma at appreciable rates, but living organisms have evolved enzymes that can speed up this reaction. Some of these enzymes include esterases which hydrolyze esters, peptidases which hydrolyze protein peptide bonds, and phosphatases which hydrolyze phosphoester bonds.

Chlorine metabolism: While humans are the main generators of organic chlorines in terrestrial environments, producing chemicals and legacy pollutants such as polychlorinated biphenyls (PCB’s) and insecticides (Figure 6), some living organisms produce chlorinated organic compounds as well, particularly those in aquatic environments. Gribble (1996) discussed “the diversity of natural organochlorines in living organisms” and offered examples, including a chemical produced by the freshwater fungus Kirschsteiniothelia sp., 3,3’-oxybis(2,4-dichloro-5-methylphenol) (Figure 7).
Organochlorines or chlorinated organic molecules are chemicals containing a C-Cl bond. The presence of chlorine on the molecule tends to make it more lipophilic and more difficult to degrade via phase I biotransformations or energy metabolism by microorganisms. These two characteristics together, high lipophilicity and persistence, promote retention of these chemicals within biological tissues and can lead to toxic buildup. Removing chlorines from organic molecules, dehalogenation, is energetically less favorable than removing common heteroatoms such as sulfur or nitrogen (Dugat-Bony, 2016). The enzymes that detoxify organochlorines are rare in life; they are usually relegated to microorganisms that use compounds for energy metabolism. Dugat-Bony, et al. (2016) described various methods used to dehalogenate the organocholrines which overlap with other detoxification processes described above. Monooxygenases such as cytochromes P-450 can both oxidatively or reductively eliminate chlorine. Glutatione S-transferase (see next section, phase II biotransformations) can reductively eliminate chlorine. Detoxification of organochlorines are undoubtedly useful for the host microbe, but they have also been studied as potential solutions to human environmental contamination involving persistent organochlorine pollutants (Jugder, 2016).


Biotransformations, Phase II
[edit | edit source]Phase II biotranasformations are characterized by additions, called conjugations, of small bio-molecules to the xenobiotic such as sugars and amino acids. These additions, larger in size than hydroxyl additions from phase I oxidations, both potentially increase the hydrophilicity of the toxicant and potentially block the molecule from its site of toxic action by changing how it moves through tissues and cell membranes. While Phase II does not always follow phase I, the functional groups created in phase I transformations such as a hydroxyl –OH group, can be used as a target for conjugation reactions of phase II biotransforamtions. Conjugations are usually more effective than phase I biotransformations in regards to increasing hydrophilicity and decreasing toxic effects. Many examples of conjugations can be found including methylations and acetylation (Figure 8); however, this chapter focuses on the most well studied: glucuronidation, sulfonation, and glutathione conjugation.

Glutathione: The glutathione S-transferases (GST) is a highly conserved conjugation enzyme family. Glutathione, a tripeptide capable of acting as a reducing agent for phase I biotransformations on its own, can be used with a GST to bind with a xenobiotic via the sulfur group on the cysteine moiety of a reduced glutathione molecule. This greatly increases the polarity and molecular weight of most target substrates, allowing them to be excreted with water soluble waste. Stenersen et al. (1986) investigated nine different animal phyla, examining both terrestrial and aquatic organisms for the presence and activity of GST. They studied aquatic and non-aquatic vertebrates, insects, crustaceans, and mollusks and found that nearly all the animals had GST activity, though activity was significantly higher for terrestrial organisms than for aquatic life. As described by Edwards, et al. (2005), plants have a small subset of water-soluble GST enzymes. Glutathione is very important for the glutathione-ascorbate cycle and reduction of hydrogen peroxide in plants, and it can also play a part in plant defense against pathogens.
Glucuronidation: Sugars are a common biomolecule and are highly water soluble, making them ideal conjugates to increase the solubility of a xenobiotic when carbohydrates are in abundance. Glucuronic acid, a derivative of glucose, is a major cofactor of phase II biotransformations and are used by the enzyme family: uridine 5’-diphospho-glucuronosyltransferase (UDP-glucuronosyltransferase, UGT). Glucuronic acid itself is initially transformed into uridine diphosphate glucuronic acid which is the sugar derivative covalently bonded to another biomolecule through a diphosphate linkage. The diphosphate raises the energy of the molecule (similar to why Adenosine triphosphate is used as energy by the cell) which allows the UDP-glucuronosyltransferase enzyme to proceed with the conjugation by releasing the energy stored in the phosphate linkage of the cofactor. In this way the cofactor acts as both conjugate and energy source (Klaassen, 2013). Enzymes without active sources of energy drastically slowdown in response to temperature decline. UDP-glucuronosyltransferase is also affected by temperature, with an optimal range dependent on the organism and its environment, but not to the same extent due to energetics. As such, UDP-glucuronosyltransferase is helpful to aquatic organisms that experience temperature changes in the water. HÄNNINEN, et al. (1984) describe how rainbow trout lose CYP-450 enzyme functionality at colder stream temperatures, but maintain the use of UDP-glucuronosyltransferase to allow continued detoxification processes in colder climates.
Sulfonation: Sulfates can be conjugated to open hydroxyl groups on xenobiotics. These are performed by sulfotransferases which take a sulfonate group (SO3-) from the cofactor 3’-phosphoadenosine-5’-phosphosulfate, which is a modified nucleotide similar to ATP (adenosine triphosphate) but with a sulfate instead of a phosphate (Figure 9). Sulfonation is an important way to biotransform the active estrogen, 17β-estradiol. Exposure to exogenous estrogens can lead to deformations of the gonads in aquatic organisms. Wang et al. (2007) explored what can happen when the sulfonation of these estrogens is blocked by other environmental contaminants. The authors found that polychlorinated biphenyls (PCBs) with hydroxyl groups (OH-PCBs) inhibited the activity of sulfotransferases to sulfonate 17β-estradiol. In this case, products of phase I biotransformations (OH-PCBs) were detrimental to the biotransformation of other xenobiotics (estrogen), essentially making the PCB more toxic or toxic in a different way. See the following section for more examples of biotransformations increasing the toxicity of a xenobiotic.

Biotransformations that Increase Toxicity
[edit | edit source]Biotransformations typically result in changing a xenobiotic so that it will not harm the organism. The range of xenobiotics that can affect an organism are diverse however, and the same biotransformation that causes one xenobiotic to become harmless could transform a different xenobiotic into something much more harmful. A human example is alcohol dehydrogenase. Ethanol, which can have harmful effects on the body or behavior on its own, undergoes an oxidative biotransformation catalyzed by alcohol dehydrogenase or other enzymes (Figure 10). The ethanol becomes ethyl aldehyde (or acetaldehyde) which is toxic to the liver and other organs (NIH NIAAA, 2007). The aldehyde is further oxidized to acetic acid (the tangy part of vinegar) which is much less toxic. A xenobiotic that becomes more toxic after a biotransformation is called a pro-toxin. The above example illustrates that alcohol is both a toxin and pro-toxin for humans.

An endangered fish species found in Mexico’s freshwater environments, Chirostoma riojai, is an example. Vega-Lo´pez et al. (2011) did a study on how naturally occurring halomethanes could be affecting this species. Halomethanes are single carbon molecules with some number of halogens, and occur naturally in water. The halomethanes underwent biotransformation with a subset of cytochrome P450s and were oxidized (see section on chlorine metabolism). The oxidized halomethanes caused oxidative stress to the fish, which can further lead to oxidative damage. The oxidation caused the xenobiotic to become more reactive and increased the exposure risk. This is an occasional downside to the broad specificity of some enzymes such as mix function oxidases.
Enzyme Induction
[edit | edit source]Types of pollutants and xenobiotics can vary according to location and time. Keeping a suit of enzymes to answer every potentially harmful xenobiotic would be energetically expensive and potentially counterproductive when there is only a finite subset of xenobiotics to address at any one time. The key to surviving and thriving is to have the correct enzymes in place when needed. Enzymes are proteins which are produced constantly by reading DNA. Cells self-regulate which DNA genes are being read and how much protein to create, depending on signals that tell the cell what is currently needed. Genes corresponding to detoxification enzymes can be turned on or “upregulated” by a process called enzyme induction. When an organism encounters a particular xenobiotic it signals a response to induce the production of detoxification enzymes to address that specific xenobiotic or closely related group. This process is not instantaneous and means that when encountering a new xenobiotic, it will likely be harmed. If an organism survives the initial encounter it will induce the production of enzymes to protect itself from subsequent encounters, similar to the immune system’s reaction to a virus. This could result from switching food sources where a new plant food is high in secondary metabolites--in this case the enzymes induced may be of general detoxifying function. A review of biotransformation systems in fish by Chambers et al. (1976) compared the prevalence of enzyme induction of mixed function oxidases between mammals and fish. Fish were found to upregulate these enzymes following a change in diet or pesticide exposure, but at lower levels than that found in mammals.
Many of the enzyme groups discussed in phase I and II biotransformations have some members that act broadly on a wide range of molecules. Mixed function oxidases are named for their broad specificity and have more than one function. More specific enzymes can be induced once a xenobiotic is encountered, and they may possess more potent activity due to their specialization. Hence, organisms can balance the tradeoff between specificity and activity while maintaining the highest chance of evolutionary success.
References
[edit | edit source]Bend, John R., Margaret O. James, and Patrick M. Dansette. "In vitro metabolism of xenobiotics in some marine animals." Annals of the New York Academy of Sciences 298.1 (1977): 505-521. Bernhardt, Rita. "Cytochromes P450 as versatile biocatalysts." Journal of biotechnology 124.1 (2006): 128-145.
Bursell, E. (1967). The excretion of nitrogen in insects. In Advances in Insect Physiology (Vol. 4, pp. 33-67). Academic Press.
Chambers, Janice E., and James D. Yarbrough. "Xenobiotic biotransformation systems in fishes." Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 55.2 (1976): 77-84.
Dugat-Bony, Eric, Pierre Peyret, and Corinne Biderre-Petit. "New Insights into the Microbial Contribution to the Chlorine Cycle in Aquatic Ecosystems." Lake Pavin. Springer, Cham, 2016. 285-306.
Edwards, Robert, and David P. Dixon. "Plant glutathione transferases." Methods in enzymology 401 (2005): 169-186.
Erickson, Russell J., and James M. McKim. "A simple flow‐limited model for exchange of organic chemicals at fish gills." Environmental Toxicology and Chemistry: An International Journal 9.2 (1990): 159-165.
Evans, David H. “The Fish Gill: Site of Action and Model for Toxic Effects of Environmental Pollutants.” Environmental Health Perspectives, vol. 71, 1987, pp. 47–58. JSTOR, www.jstor.org/stable/3430412.
Gribble, Gordon W. "The diversity of natural organochlorines in living organisms." Pure and applied chemistry 68.9 (1996): 1699-1712.
HÄNNINEN, OSMO, et al. "Glucuronidation and glucosidation reactions in aquatic species in boreal regions." (1984): 13-17.
Jugder, Bat-Erdene, Haluk Ertan, Susanne Bohl, Matthew Lee, Christopher P. Marquis, and Michael Manefield. "Organohalide respiring bacteria and reductive dehalogenases: key tools in organohalide bioremediation." Frontiers in microbiology 7 (2016): 249.
Klaassen, Curtis D., and Mary O. Amdur, eds. Casarett and Doull's toxicology: the basic science of poisons. Vol. 1236. New York: McGraw-Hill, 2013.
McKim, J., P. Schmieder, and G. Veith. "Absorption dynamics of organic chemical transport across trout gills as related to octanol-water partition coefficient." Toxicology and applied pharmacology 77.1 (1985): 1-10.
Menn, Julius J. "Comparative aspects of pesticide metabolism in plants and animals." Environmental Health Perspectives 27 (1978): 113-124.
Moktali, Venkatesh, et al. "Systematic and searchable classification of cytochrome P450 proteins encoded by fungal and oomycete genomes." BMC genomics 13.1 (2012): 525.
NIH NIAAA (2007): Alcohol metabolism: an Update https://pubs.niaaa.nih.gov/publications/aa72/aa72.htm
OpenStax. “Anatomy and Physiology.” Victoria, BC: BCcampus. OpenStax (2013) Chapter 26.2. https://opentextbc.ca/anatomyandphysiology/chapter/26-2-water-balance/
Stegeman, John J., and John J. Lech. "Cytochrome P-450 monooxygenase systems in aquatic species: carcinogen metabolism and biomarkers for carcinogen and pollutant exposure." Environmental health perspectives 90 (1991): 101-109.
Stenersen, J., et al. "Glutathione transferases in aquatic and terrestrial animals from nine phyla." Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 86.1 (1987): 73-82.
Timmermans, K. R., W. Stolte, and H. J. W. De Baar. "Iron-mediated effects on nitrate reductase in marine phytoplankton." Marine Biology 121.2 (1994): 389-396.
Wang, Li-Quan, and Margaret O. James. "Sulfonation of 17β-estradiol and inhibition of sulfotransferase activity by polychlorobiphenylols and celecoxib in channel catfish, Ictalurus punctatus." Aquatic toxicology 81.3 (2007): 286-292.
Vega-López, A., Carrillo-Morales, C. I., Olivares-Rubio, H. F., Domínguez-López, M. L., & García-Latorre, E. A. (2012). Evidence of bioactivation of halomethanes and its relation to oxidative stress response in Chirostoma riojai, an endangered fish from a polluted lake in Mexico. Archives of environmental contamination and toxicology, 62(3), 479-493.
Vega-López, Armando, et al. "Relations of oxidative stress in freshwater phytoplankton with heavy metals and polycyclic aromatic hydrocarbons." Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 165.4 (2013): 498-507.
Glossary
[edit | edit source]Aquatic organism – An organism that spends a part of its life-cycle (e.g. larval stage) or a whole life-cycle in the water. For this chapter it includes both freshwater and saltwater environments. Some literature uses the term “marine organism” for saltwater environments.
Xenobiotic – A molecule that originates from outside an organism that is not naturally produced (synthetic) or expected to be present in the organism. They are not always toxic (many are beneficial) but this chapter will use the term to refer to a potentially toxic substance.
Biotransformation – The process of a living organism changing a chemical. A biotransformation is often carried out by an enzyme.
Enzyme – A protein that catalyzes a chemical reaction. This means it facilitates and speeds up chemical reactions. Enzymes are unchanged after the reaction, allowing them to repeat the process; but, may be limited by the availability of cofactors.
Cofactor – A biomolecule that is required by an enzyme to function. In biotransformations the cofactor is used up by the enzyme to change the xenobiotic.
Oxidation/Reduction – Terms for chemical reactions in which electrons are transferred from one molecule to another. These reactions go in pairs because if one molecule is gaining electrons another must lose them. The names in Phase I biotransformations refers to the effect on the xenobiotic. I.e. “oxidation reactions” are where the xenobiotic is oxidized (looses electrons); the cofactor is reduced and the enzyme stays the same.
Enzyme Induction – The upregulation of transcription for a certain protein due to internal signaling in an organism. In this chapter it refers to the upregulation of detoxifying enzymes after a poisoning to counteract further poisonings.
Conjugation – The chemical process of joining two molecules together.
Toxicokinetics – Movement of a toxic chemical through a living organism. This includes absorption, distribution, metabolism and excretion. Toxicokinetics is sometimes referred to as, “How the body acts on the chemical.”
Toxicodynamics – The way a toxic chemical exerts its toxic effect. How the toxin interacts with the target site of action. Toxicodynamics is sometimes referred to as, “How the chemical acts on the body.”
Pollutant – A substance in the environment at least in part as a result of human origin or activity which has deleterious effect on living organisms.
Toxin – A chemical that causes harm to an organism. Any chemical can be a toxin depending on the dose/concentration and exposure.
Metabolism – The breaking down or conjoining of molecules by an organism.
Energy metabolism – Metabolism involved in generating energy for an organism to live; it is also called “catabolism.”
Co-metabolism – Metabolic processes used to break down molecules, but do not yield energy for the organism.
Conserved (evolution) – A characteristic of a gene or morphological feature. Conserved genes/morphologies stay the same or relatively unchanged across different branches of the evolutionary tree. For example, if there is an enzyme that is the same in rainbow trout and humans, that enzyme and the corresponding gene is “conserved between rainbow trout and humans.” If the same enzyme is present in many other taxa as well, it would be called “generally conserved” or “highly conserved.”
Chapter 3: Micro-plastics: An Emerging Pollutant in an Aquatic Ecosystem
Chapter 3: Microplastics: An Emerging Pollutant in an Aquatic Ecosystem
[edit | edit source]Introduction
[edit | edit source]Introduction to Microplastics
[edit | edit source]If you were to causally glance at your surroundings, chances are you would see a multitude of products that are made of, or utilize plastics. Plastics are long chains of polymers synthesized from both organic and inorganic materials such as carbon, hydrogen, silicon, oxygen, and chloride which are usually acquired from natural resources such as natural gas, oil, and coal (Ivar do Sul et al., 2013). Plastic is considered to be a fairly recent invention. In 1907, the first plastic Bakelite was synthesized. However, it wasn’t until the 1940s that plastic production began in earnest (Cole et al., 2011). Copious amounts of plastics have been synthesized and released into the environment. One of the major draws of plastic is its durability, which may ultimately lead to its downfall. Tons of plastic pieces find their way each year into various watersheds, and subsequently the oceans. Many studies have demonstrated the hazards of plastics to aquatic wildlife. (Figure 2 & 3)


These studies focus on macroplastics: plastics that are easy to see with the naked eye. However, plastics come in a variety of sizes: macroplastics, microplastics, and nanoplastics. There is no standard definition for microplastics, and thus the term “microplastic” is controversial. The term does not refer to the standard micro unit as seen in the International System of Units (1-999 μm). A microplastic can be a particle that has a diameter of < 10 mm, or < 5 mm, or 2-6 mm, or <1 mm (Cole et. al., 2011) (Figure 4). Nor is there a set definition for nanoplastics (plastic particles that are smaller than microplastics) and that absence further complicates matters. Again, the term “nano” in nanoplastic does not refer to the nano size typically seen in a laboratory setting. For this chapter, the term microplastic means any plastic particle that is < 5 mm in diameter but greater than 100 nm. The term nanoplastic refers to plastics that are between 1-100 nm (Jovanović, 2017).

Types of Microplastics in the Environment
[edit | edit source]There are two major categories of microplastics: primary and secondary. Primary microplastics are plastics manufactured in the microplastic size range and often used in cosmetics, facial cleansers, facial exfoliants, air blasting media, and even medicine. Occasionally, these microplastics are called micro-beads or micro-exfoliants. Primary microplastics can also include virgin plastic production pellets. Secondary microplastics are microplastic particles that have fragmented from macroplastics (Cole et al., 2011). Common synthetic plastics used today include polypropylene, polyethylene, polystyrene, polyvinyl chloride, polyethylene terephthalate, and as well as others (Ivar do Sul et al., 2013) (Figure 5).

Quantification and Location of Microplastics Worldwide
[edit | edit source]As previously mentioned, the first major plastic, Bakelite, was produced in 1907; however, the mass production of plastic containing compounds began much later in the 1940s (Cole et al., 2011). Since then, the amount of plastic produced worldwide has continued to increase at a rapid rate. In 2017, it was estimated that over 350 million metric tons of plastic were produced, and since then plastic production has increased even further (PlasticsEurope, 2018). Of the plastic manufactured each year, roughly up to 10% of it will subsequently end up in aquatic environments where it will reside for extended periods of time. Thus, the quantify of plastic accumulating in aquatic environments is continuously increasing. By 2050 it is estimated that the amount of plastic present in the oceans will either be equal to or greater than the total weight of fish in that same environment (Jovanović, 2017). In 2014, van Sebille, et al. estimated the amount of microplastics on the surface of the ocean to be between 15-51 trillion particles, together weighing between 93-236 thousand metric tons.
Microplastic particles have been located worldwide, even in areas that should be devoid of plastics. Additionally, the type of microplastic present in such environments does not substantially differ from region to region: it is possible to find polystyrene microplastics everywhere (Koelmans, et al., 2016). Microplastics are present at various depths in the water column. Typically, microplastics such as polyethylene and polypropylene are very buoyant and may remain on the surface of water. However, it is also possible for other microplastics such as polyvinyl chloride to be less buoyant or for the buoyancy of the particle to decrease or increase due to surface growth of microbial films. These microplastics particles may then be suspended in the water column or be located on, or in the sediment (Ivar do Sul, et al., 2013).
Models used to estimate the prevalence of microplastics in the ocean often focus on microplastics located on the surface of the water. As such, they capture a brief snapshot of microplastic presence in one specific area of the water column. However, vertical mixing of particles in the water column may occur as a result of wind (Ivar do Sul, et al., 2013.) Additionally, more microplastic particles enter into an aquatic ecosystem after storms, flooding, or other types of extreme weather (Cole, et al., 2011). This makes it difficult to obtain an accurate image of how many pieces of microplastic are present on the ocean surface. Nevertheless, there are areas more or less abundant in the number of microplastic particles compared to other areas. For instance, there are fewer microplastics present in the tropics above 45ᵒN and below 45ᵒS, and the remote coastline off Western Australia. The highest concentrations are found in centers of subtropical gyres located in North Atlantic and North Pacific regions. The highest concentration in subtropical gyres is estimated to be 108 particles km-2. The highest counts of microplastics are located in Mediterranean and North Pacific basins while microplastics present in the North Pacific basin contain the largest total mass (Figure 6). This is likely due to the vast area of the North Pacific basin and the large quantity of plastic waste entering the ocean from the coastlines of the United States and Asia. Ultimately, the majority of microplastics lie in regions of low concentration. Further data is needed to verify these estimates, especially in less analyzed regions such as South America (van Sebille, et al., 2015).

Methods Used to Study Microplastics
[edit | edit source]Various methods are used to quantify the number of microplastics present in an aquatic environment. These include beach combing, sediment testing, biological sampling, marine observational surveys, and marine trawls. Beach combing involves collecting, identifying, and quantifying all litter items on the shore of a specific coastline region, and If done periodically it can illustrate the debris accumulation during a specific time frame. This process is best suited for quantifying macroplastics and occasionally larger microplastics. For example, it is possible to quantify plastic resin pellets called Mermaid’s Tears while beach combing. Sediment testing involves taking sediment samples from the sediment of an aquatic environment and quantifying the amount of microplastics in benthic regions. Biological sampling involves quantifying the amount of microplastics consumed by aquatic organisms (See Section C for examples). Marine observational sampling is conducted by divers or onboard observers who record the size, location and types of plastic observed in the water. However, the small nature of microplastics allows them to be unobservable and ergo, undetected. Marine trawls constitute one of the more popular methods used to capture, identify and quantify microplastics where fine meshes or nets are dragged behind boats. It is common to use a plankton net in these studies; the size of the net can vary between 0.1 mm to 0.5 mm (Cole et al., 2011; van Sebille et al., 2015).
Microplastics are typically quantified either by sediment testing or marine trawls. It is important to filter out impurities from the samples and to promote the migration of microplastics to various solution surfaces after samples have been collected (Figure 7). Water samples are run through a coarse filter to remove microplastic particles and other larger contaminants. Meanwhile, sediment samples are slurried with saline water to promote the migration of microplastics to the sample’s surface. Additionally, mineral salts may be added to either the water sample or the slurried sample to increase water density, as more microplastics may float to the surface of a sample when water density is greater. Evaporation is then commonly used to concentrate the microplastic particles at the surface of the sample. Subsequently, regions of the samples with microplastics will have been isolated and can be removed for quantification. Following removal, microplastics in surface water samples can be stained with a lipophilic dye such as Nile Red in order to increase ease of visualization. Microbiota that are present in the solution or on the microplastic pieces are not stained with the dye. Sometimes hot dilute mineral acid is used to remove any additional biomass. Finally, the microplastic pieces identified and quantified using various types of microscopy such as Raman spectroscopy, FTIR spectroscopy, electron microscopy, and optical microscopy (Andrady, 2011).

Ingestion of Microplastics
[edit | edit source]Plastics are ubiquitous: it is not uncommon to find bits and microplastic pieces in and around aquatic wildlife. Haunting images of turtles trapped inside plastic have invaded the media for years. Other images have shown tons of plastics almost spilling out of the guts of aquatic birds or whales. (Figure 8) In fact, at least 44% of all marine bird species have ingested plastics (Andrady, 2011).
Both macroplastics and microplastics have been ingested by a variety of aquatic wildlife at different trophic levels. Microplastics do not discriminate against species (although some species may discriminate against microplastics--e.g. selective filter feeders) and have been found in and around both invertebrates and vertebrates. (Table 1)
Table 1: Examples of species that have ingested microplastics
| Class of animal | Type of Animal | Name | Scientific Name |
| invertebrate | Insect | Water Strider | Halobates micans |
| invertebrate | Insect | Water Strider | H. sericeus |
| invertebrate | Mollusk | Blue Mussel | Mytilus edulis |
| invertebrate | Crustaceans | Green Crab | Carcinus maenus |
| vertebrate | Fish | Flounder | Psuedopleuronects |
| vertebrate | Fish | Bass | Morone america |
| vertebrate | Bird | Fulmar | Fulmarus glacialis |
| vertebrate | Bird | Fledgling Cory Shearwaters | Calonectris diomedea |
Ingestion of Microplastics by Invertebrates
[edit | edit source]Colonies of microscopic organisms can grow microbial biofilms on various microplastics, and these biofilms can accumulate on microplastics in less than a week. As a result, species of invertebrates and algae have been able to grow on the surfaces of these particles (Cole, et.al, 2011). Additionally, insects such as Halobates micans and H. sericeus use plastic pellets as oviposition sites (Majer et al., 2012; Goldstein et al., 2012). For example, the numbers of eggs of H. sericeus juveniles and adults in the North Pacific Ocean were significantly and positively correlated with the number of microplastics present. Furthermore, 34% of all plastic pellets found in the western Atlantic had eggs attached to them (Barnes, et al., 2009).
Laboratory experiments have been used often to highlight how invertebrates ingest microplastic particles. In 2004, the first experiment to test microplastic ingestion by barnacles (Semibalanus balanoides), amphipods (Orchestia gammarellus), and lugworms (Arenicola marina) was performed, and demonstrated that these three species could ingest microplastics (Thompson et al., 2004). Other studies have tested various mollusks, crustaceans, annelids, and echinoderms. For example, one of the more commonly studied mussels in regard to microplastic ingestion is Mytilus edulis. This species ingests and accumulates microplastics within 12 hours of exposure. Interestingly, not only were microplastic pieces found in the intestines, they were also present in the gills and hemolymph of the mussels (Browne et al., 2008; von Moos et al., 2012). Additionally, microplastics found in these mussels were later transferred to a higher trophic level: Carcinus maenus. As a result, microplastic particles have been found in the hemolymph and various other tissues of these crabs (Farrell and Nelson, 2012). Thus far, studies of microplastic ingestion by benthic crustaceans have been limited. However, 83% of sampled lobsters in the Clyde Sea had microplastic particles in their stomachs. Additionally, lobsters were shown to ingest microplastics within 24 hours of being exposed (Murray and Cowie, 2011). Microplastic ingestion has even been identified in humboldt squids (Dosidicus gigas), and sea cucumbers (Holothuria) have been known to preferentially feed on nylon and PVC fragments over sediment grains (Braid et al., 2012; Graham and Thompson, 2009). Finally, many types of zooplankton have also been studied for the possibility of microplastic ingestion, and It was confirmed that many taxa do indeed ingest the particles (Cole et al., 2013). The possibility of ingestion of microplastics by various types of invertebrates seems endless.
Ingestion of Microplastics by Vertebrates
[edit | edit source]Microplastic particles have been discovered in the guts of various vertebrates since 1972. Some of the first particles detected in larvae and juvenile fish were found in Psuedopleuronects flounder, while some of the first particles detected in adult fish were found in Morone america and Protonus evolans (Carpenter et al., 1972; Hoss and Settle, 1990). The potential for possible ingestion of microplastics became concerning when a study (Boerger et al., 2010) found synthetic fragments in 35% of planktivorous fish located in the North Pacific Central Gyre. A study (Lusher et al., 2013) that evaluated demersal and pelagic fish in the coastal waters around the United Kingdom found further evidence of microplastic ingestion among various species, with 36% of the species tested having ingested fibers and microplastic fragments. Additionally, mesopelagic fish located in the North Pacific were found to have ingested microplastic particles and fibers (Davison and Asch, 2011). To further illustrate the enormity of the problem, 40% of lantern fish tested in the Marianna Islands--a region that contains less microplastic particles--were found to have ingested microplastics, further illustrating how ubiquitous microplastics are in terms of ingestation (Van Noord, 2013).
Microplastic ingestion by vertebrates is not limited to the marine environment. Studies have found these particles in fish living in estuaries as well. For example, a study conducted in the western South Atlantic Ocean reported that mojarras (Gerreidae), estuarine drums (Sciaenidae), and catfishes (Ariidae) all ingested microplastics. All three species feed on or just below the surface of the sediment, and the most popular ingested microplastic was blue nylon thread. They most likely ingested the microplastic particles accidentally through normal suction feeding, eating contaminated prey, and/or by actively feeding on plastics with biofilm (Ramos et al., 2012; Dantas et al., 2012; Possatto et al., 2011). Microplastics have been found ingested by fish in all areas of the world and at all levels of the water column.
Although not commonly thought of as aquatic vertebrates, shore birds have also been known to ingest microplastics. In fact, the ingestion of plastics by birds has been studied for the past four decades, although many of these studies did not distinguish between microplastics and macroplastics. Microplastic pellets have been found in the guts of migratory petrels--shearwaters and prions--in the Atlantic and south-western Indian Ocean among others since the 1980s. Plastic pellets and other plastic fragments were found in 80% of two Fulmarus glacialis colonies in the Canadian Arctic (Provencher et al., 2009). Additionally, the Fulmarus glacialis species has been monitored in both the North Sea and the Netherlands for the past 30 years. During this time, it was discovered that while the number of plastic pellets decreased by 50% in 20 years, the number of plastic fragments tripled. Interestingly, juvenile Fulmarus glacialis ingested more plastic than the adults. It was also noted that there was an increased presence of ingested microplastics in areas near highly industrialized areas specializing in either shipping or fishing (Van Franeker et al., 2011; Kühn and van Franeker, 2012). Furthermore, around 90% of all fulmars sampled in the North Pacific Ocean had ingested microplastics (Avery-Gomm et al., 2012). Microplastics were found ingested by birds located in Iceland as well; but overall, fewer had ingested the particles. Thus, it has been hypothesized that birds further north are less likely to be contaminated by microplastics because there are fewer microplastics available on the surface of the water in these regions (Van Franeker et al., 2011; Kühn and van Franeker, 2012). This hypothesis was also proposed by a study done on the ingestion of microplastics by Uria lomvia in Nunavut, Canada in which 11% of the birds investigated had ingested microplastics; however, the authors hypothesized that the low number may be due to these birds feeding below the water surface and are therefore less likely to ingest floating plastics (Provencher et al., 2010). It is interesting to note that birds never exposed to marine environments may ingest microplastics as well. For example, 83.5% of fledgling cory shearwaters (Calonectris diomedea) were found to have ingested microplastics. These chicks were exposed to pieces of microplastic by eating the regurgitated food from their parents (Rodríguez et al., 2012). Other bird species known to ingest microplastics include Larus glaucescens, P. nigripes, Phoebastria immutabilis, among others (Lindborg et al., 2012; Gray et al., 2012; Avery-Gomm et al., 2013).
Research related to ingestion of microplastics by marine mammals is limited; however, it has been shown by analyzing their scat that marine mammals do indeed ingest microplastics. For example, fur seal scat (A. gazelle and Arctocephalus tropicalis) collected on Macquaire Island contained plastic fragments and pellets that were 2-5 mm in size. These plastics were believed to have come from the animal’s prey that ingested microplastics present in or on the water (Eriksson and Burton, 2003). Future studies will likely highlight the presence of microplastics found in aquatic mammal intestines.
Degradation of Microplastics
[edit | edit source]Macroplastics eventually become microplastics, and microplastic pieces eventually degrade into nanoplastics. It is estimated that it will take approximately 320 years for a 1 mm sized microplastic to degrade into a nanoplastic within a marine environment (Koelmans et.al, 2015). Degradation is the chemical process by which the average molecular weight of a polymer is reduced. Plastic degradation to any size will eventually weaken the material and the particles may become so weak they fall apart into a powdery substance subsequently degraded further. Plastic is said to have mineralized when all carbon present in the polymer is converted into CO2: mineralization is the goal of degradation. The process of degradation can fall into one of five major categories: thermal degradation, photodegradation, thermooxidative degradation, biodegradation, and hydrolysis.
Different types of Degradation
[edit | edit source]Thermal degradation is the degradation that occurs via high temperatures. This is not considered a mechanism by which microplastics degrade in the environment. Photodegradation is the process by which light, usually sunlight, degrades a material. UV-B is the primary component in photodegradation. It is also considered to be one of the fastest methods of degradation for microplastics, working best for particles that are exposed to air or lying on a beach; however, the degradation rate decreases when the microplastic particles enter the water. Additionally, photodegradation is often the precursor to other types of degradation, and it is often followed by thermooxidative degradation. Thermooxidative degradation is the process by which particles are slowly broken down by oxidation in moderate temperatures--a process which can continue to degrade the microplastic particle as long as oxygen surrounds the microplastic. The quantity of oxygen present decreases in an aquatic environment, and a lack of oxygen and colder temperature will often decrease the degradation rate of a microplastic. Additionally, the growth of organisms on a microplastic can decrease the rate of degradation. This process is different from biodegradation. Biodegradation is the process by which living organisms degrade microplastics and it is typically carried out by microscopic organisms; however, these organisms are rare in nature. Finally, hydrolysis is the process by which water is used to degrade a compound; it is one of the slowest in the marine environment and rarely occurs. Once again, photodegradation and thermooxidative degradation are the most common ways in which microplastic particles are degraded into smaller pieces and eventually into nanoplastics (Andrady, 2011).
Equilibrium Partitioning Equations
[edit | edit source]It is helpful to discuss the concept of equilibrium partitioning before discussing the relative toxicity of each microplastic. Chemicals often partition in either organic matter or water, as discussed in previous chapters. Additionally, toxicants can have sorption to the plastic particles themselves. However, microplastics do not follow typical equilibrium partitioning concepts: they do not have a log Kow. Instead, specialized equilibrium factors for microplastics must be used to determine how a toxicant may partition. There are many different factors that affect the overall kinetics of the equilibrium partitioning process such as the equilibrium between water-biota, water-sediment, and water-plastic. The equilibrium partitioning equation used to determine if a toxicant will sorb to a piece of microplastic (KPL) is defined as:
KPL = CPL/Cw
where CPL (μg/kg) is the concentration of the toxicant in plastic and CW is the concentration of the toxicant in water. If KPL > CPL/CW, the toxicant can desorb from plastic to water, but if KPL <CPL/CW, the toxicant will sorb from water to plastic (Koelmans, et al., 2016). Transfer of chemicals to plastic can also be defined more precisely as:
dCPL/ dt= k1CW − k2CPL
in which k1 and k2 are first-order rate constants related to the thickness of the undisturbed boundary layer surrounding the microplastic and the ability of a chemical to diffuse in an aqueous environment, in which t= time, and in which CPL /CW are the same values as previously discussed. This equation differs from the previous equation by factoring the unbound layer that surrounds the microplastic. Both equations describe whether a compound is more likely to sorb from water to the microplastic or from the microplastic to water. Thus, microplastics can serve as a way to clean up toxicants from water or a possible manner by which toxicants enter the water. This concept of equilibrium portioning also occurs inside an organism. (Figure 9). The process of toxicant movement from one source to another will continue until equilibrium is reached.

Bioaccumulation is the ability of a chemical to continuously accumulate inside an organism. Koelmans, et al. modelled the bioaccumulation of hydrophobic compounds from an environment containing plastics to an organism as follows:
dCB,t/dt = kdermCW + IR(SFOODaFOODCFOOD + SPLCPLR,t) − klossCB,t
where Kderm CW is equal to the dermal (including gills) uptake of a chemical from the water, the second term reflects the uptake of the chemical from the diet and the exchange of that chemical with plastic particles, and the final term refers to the overall loss of the chemical as a result of elimination and egestion. More specifically, Kderm (L x kg x d-1) and kloss (d-1) are first order rate constants while IR (g x g-1 x d-1) is the mass of food ingested per organism dry weight and per time. Additionally, aFOOD refers to the absorption efficiency from the diet while SFOOD and SPL refer to the mass fractions of plastic and food in ingested material. Finally, CFOOD refers to the contaminant concentration that occurs from food to the organisms itself. Once again, this equation would highlight the ability of a compound to bioaccumulate inside an organism. Koelmans, et al. (2013a, b, 2014b) assumes that the degradation of microplastics will not occur during their journey through the gastrointestinal tract. Thus, the ability for the compound to transfer from a plastic particle to the gut is dependent on the concentration of microplastic and biota lipids; the kinetics of this are as follows:
CPLR,t = [(k1GCPL − k2GCL,t) / k1G + (MPL/ML)k2G] * (1 − e − [k1G + (MPL/ML)k2G]GRT
where k1G and K2G are first-order rate constants which describe the transport of the compound between plastics and lipids inside the gastrointestinal tract. Next, GRT is equal to the gut residence time while CPL and CL,t refer to the chemical concentrations in the ingested plastic and the biota lipids at the moment when the microplastic is first ingested. Finally, MPL and ML refer to the mass of the plastic and lipid within the organism. If the term in the numerator of this equation is positive, then the chemical will transfer from the plastic to the biota lipid. When the term is negative, cleanup of the compound from the lipid will occur, and the compound will sorb to the microplastic (Koelmans, 2015).
To summarize, a chemical compound has the potential to sorb from water to the microplastic and from the lipids of an organism to a microplastic. This is known as cleaning up. Additionally, a chemical compound may sorb from the microplastic to the water or from a microplastic to the gastrointestinal tract of an organism. The tendency of a chemical to act in such a manner can be defined according to the previously mentioned equations.
Microplastic Induced Toxicity
[edit | edit source]Microplastics, as a whole, are not considered intrinsically toxic to aquatic life. However, it is possible for chemicals added to the plastic upon synthesis to later leach out of the plastic upon degradation. Leaching is the process by which a chemical leaves the microplastic and enters the aquatic environment. Additionally, microplastics have the ability to adsorb various chemicals already in an aquatic environment. Once adsorbed, it is possible for these chemicals to subsequently desorb from the microplastics. If a microplastic with an adsorbed chemical is ingested, it is possible for the chemical to desorb inside the aquatic organism and subsequently, harm the aquatic organism. It is additionally possible that the effects of desorption by contaminated particles may bio-magnify up the food chain.
Types of Toxicants
[edit | edit source]There are a variety of toxicants associated with the aquatic toxicity of microplastics. Toxicants are human-made chemicals that cause a deleterious or harmful response in an organism. The size and type of microplastic ingested by an aquatic organism may result in an adverse physical response. Thus, the properties of a microplastic can elicit a physical adverse response. Additionally, toxicants found in the aquatic environment can potentially adsorb to the microplastic. For example, hydrophobic organic compounds can adsorb to microplastics. Hydrophobic organic compounds (HOCs) are organic compounds that are more soluble in organic solutions than water. Some hydrophobic organic compounds are polybrominated diethyl ethers, polycyclic aromatic hydrocarbons, and polychlorobiphenyls. These HOCs have been found on microplastics synthesized from polystyrene, polyethylene, polyvinylchloride, and polyoxymethylene (Koelmans, 2015). Additionally, persistent organic pollutants are potential toxicants and a major concern. Persistent organic pollutants are lipophilic chemicals that prefer to concentrate on hydrophobic regions of microplastics. Some POPs include polychlorinated biphenyls, organochlorine pesticides such as DDT and DDE, and polyaromatic hydrocarbons (Cole, et al., 2011). There some chemical overlaps classified as HOCs and POPs. Finally, additives are considered possible toxicants. Additives are compounds added to plastic while it is being synthesized. Some examples of additives include flame retardants, plasticizers such as di-ethylhexyl phthalate and bisphenol A, antioxidants, and photostabilizers (Kwon, et al., 2017).
Target Sites for Toxicity
[edit | edit source]It is important to mention target toxicity sites before discussing possible toxicological effects of compounds or the physical ramifications of ingesting microplastics. Organisms are known to ingest toxicants, as mentioned in previous chapters. Thus, a major toxicity site is the gastrointestinal tract. Aquatic organisms such as fish swallow microplastics by mistaking the particles for natural prey, or swallow microplastics unintentionally by feeding on prey that either have microplastic particles inside or adhered to their surface. As previously mentioned in section C of this chapter, many organisms studied have contained microplastic particles in their gastrointestinal tract. In fish there is no correlation between the number of microparticles residing in the gut and its mass, length, and/or the species trophic level within the food web (Güven et al., 2017); therefore, microplastics do not exhibit biomagnification. It is only the fish’s location or type of habitat that shows a difference in the number of ingested microplastic particles. For example, pelagic fish ingest more microplastic particles than benthic fish. Overall, the average number of particles found in fish is typically less than 2-3 microplastics (Güven et al., 2017). It is possible for these particles to enter into the circulatory system following ingestion, but this process is extremely rare except for microplastics that measure less than 150 μm. Small amounts of ingested microplastics may also be translocated to the liver, but typically are extremely small: less than 5 μm (Jovanović, 2017).
Adverse Physical Reactions
[edit | edit source]As previously mentioned, the ingestion of microplastics can lead physically adverse responses. Ingested microplastic particles can physically block the digestive organs and interfere with the organism’s feeding process. For example, ichthyoplankton in later developmental stages can ingest microplastics, and once swallowed the particles may lead to starvation or satiation, the latter resulting in reduced growth rate, reduced ability to avoid predators, and reduced general fitness. Additionally, fish that feed on microplastic containing zooplankton require over twice as much time to consume food when compared to those that feed on microplastic-free zooplankton. Other studies on gobies have indicated that microplastic ingestion leads to decreased predatory performance.
Microplastic particles are often irregular in shape and can thus harm the intestinal lining of organisms such as fish. For example, microplastic ingestion has shown a widening of a lamina propria, vacuolation of enterocytes, increase of goblet cells, hyperplasia of goblet cells, loss of the regular serosa structure, detachment of the mucosal epithelium from the lamina propria, and a shortening and swelling of the villi. It is also possible for microplastics to cause neurological effects and metabolic changes such as the upregulation of fatty acids (Jovanović, 2017). Additionally, the presence of microplastic particles on beaches can raise beach sand temperature, affecting the ratio of male to female organisms for species that have sex determined by temperature (Ivar do Sul, et al., 2014). As a result, the population of these species may decrease across subsequent generations. Indeed, the physical presence of microplastic can have adverse effect on organisms.
Hydrophobic Organic Compounds
[edit | edit source]Microplastics, themselves, are not intrinsically toxic, as previously mentioned. Most of the concern surrounding microplastic presence in the ocean reflects the fact that toxicants may adsorb to plastic and then desorb inside an organism, or leach from microplastic into the environment and/or an organism and cause a deleterious response. HOCs as previously mentioned comprise a set of compounds that may adsorb to plastics (Figure 10).

It has been shown that HOCs on microplastics may indeed be a source of HOCs in an aquatic environment; however, whether HOCs on microplastics cause a more deleterious response than other HOCs found in the environment is more controversial. Some studies argue that the affinity of HOCs for plastics is very high. On average, the half-life for a HOC to desorb to a microplastic particle is around 2 years. However, the smaller the microplastic, the shorter the half-life of the HOC. Nevertheless, a different picture begins to emerge when one factors in the ratio of microplastics to organic matter or other carrier media for HOCs Some of these other carrier media types have equilibrium partition coefficients that are equal to that of microplastic particles. Thus, the potential toxicity from HOCs absorbed to microplastics can only occur if the concentration of HOC on the microplastic is greater than the concentration of HOCs present in the water or in an organism. Nevertheless, the amount of microplastic particles in an aquatic environment such as the ocean is too small to cause a meaningful redistribution of HOCs from the ocean to microplastic particles themselves. For example, when comparing the transfer of HOCs to microplastics or organic black carbon, it was determined that the partition coefficient for black carbon is twice as high as the partition coefficient of microplastics. Thus, it is twice as likely that a HOC would sorb to organic black carbon than to sorb to a piece of microplastic. Usually, HOCs are present in the water. Therefore, the most likely route of exposure for an organism is by ingesting contaminated water rather than ingesting contaminated microplastics (Koelmans, et al., 2016).
Persistent Organic Pollutants
[edit | edit source]Persistent organic pollutants also have the potential to adsorb to pieces of microplastic, causing concern that once ingested these POPs may desorb from the microplastic and cause harm to the organism (Figure 11). POPs have very large partitioning coefficients in favor of microplastics; therefore, microplastics have a tendency to clean up POPs from aqueous solutions, but the amount of macroplastics present in the environment is quite small compared to that of POPs. Hence, not all POPs can be removed via the presence of microplastics.
A study by Tueten, et al. indicated that POPs have a very slow desorption process off of microplastics. Although it is possible for POPs to already be present in a microplastic particle and leach out of the particle, the number of particles for which this could occur is negligible compared to the amount of POPs that enter the aquatic environment from other sources. Nevertheless, microplastics tend to have a cleaning up effect on POP. If a microplastic particle is ingested inside an organism whose gut contains substantial levels of POPs, the microplastic will serve as a sink for the POPs and actually decrease the level of POPs inside the organism (Andrady, 2011; Koelmans, 2015).

Whether toxicants such as HOCs and POPs that can adsorb to microplastic particles and then later desorb are toxic is in direct correlation to the amount of that toxicant present in the environment. A bioaccumulation of the compound inside an organism might occur if the microplastic contains the only source of the chemical. Nevertheless, it is extremely unlikely that microplastics would contain the only source of these toxicants. Therefore, the possibility of bioaccumulation via a microplastic vector decreases as the chemical gradient shifts towards the adsorption of these toxicants by microplastics. As a result, microplastics are more likely to serve as cleaners than contributors to the bioaccumulation of HOCs and POPs (Koelmans, 2015; Koelmans, et al., 2016).
Additives
[edit | edit source]Finally, a major concern is that additives might act as toxicants to aquatic organisms. Since these chemicals may leach out of plastic, there may be more chemicals present in the plastic during the beginning stages of leaching than in the water or organism. Once the process of leaching has commenced, it could continue until a chemical equilibrium gradient has been established with the microplastic and its surrounding solution. Some laboratory studies have indicated that leaching of an additive from microplastic can occur inside an organism. Other model studies indicate that the number of additives that leach out of a microplastic in an organism would be negligible.
To further complicate matters, compounds considered to be additives have a widespread range of characteristics. Some of them are very hydrophobic while others are more hydrophilic. Thus, the probability of an additive causing a toxicological effect varies from additive to additive. For example, additives with long alkyl groups are not likely to bioaccumulate, but those such as benzotriazole UV stabilizers may do so. The ability for additives to cause a deleterious effect on aquatic organisms has been documented, but it is unclear whether the effects were due to additives that came directly from microplastics or from other sources (Kwon, et al., 2017).
Problems Regarding Microplastic Research and Future Directions
[edit | edit source]The field of microplastic research that has investigated microplastics’ potential toxicity to aquatic environments is relatively new. Thus, there are numerous problems to consider when designing an experiment or reading papers on the topic. First and foremost, there is not a standard definition for microplastics. The definition changes according to the researcher who attempts to redefine it, making it difficult to fully grasp the topic. Additionally, there are no standardized techniques on how to measure, study and analyze microplastics, or their effects on the aquatic environment. Plankton nets are commonly used, but they fail to collect some of the smaller microplastics. Furthermore, current data regarding the toxicity of microplastics or possible toxicants adsorbed to them is controversial. Some experiments demonstrate that the presence of microplastics and their toxicants such as HOCs, POPs, and additives do cause a deleterious response. Other experiments and models indicate there is little to no effect caused by microplastic particles when compared to the presence of the same chemicals in water. Furthermore, the problem of microplastic research is exacerbated by the fact that it is not clear what the actual environmental concentration (AEC) of microplastics is, and that the AEC is highly variable both on a macroscale (geographically) and on a microscale (water surface, water column, and sediment).
One of the difficult aspects of any aquatic toxicity tests is selecting the type of test to perform. A variety of tests can be performed in microplastic research such as laboratory tests, model systems, and field tests. However, each of these tests has its positive and negative attributes. Often, the doses used in laboratory tests do not reflect actual or estimated environmental concentrations and far exceed worst-case scenarios. These tests also focus on only one species and ignore intricate aspects of the ecosystem. Furthermore, modeling studies often fail to be evaluated with actual data; thus, they may not reflect what is actually occurring in the environment. Finally, field-based studies are difficult to perform. They require considerable time, money, and preparation. When preformed, it is often difficult to extrapolate data regarding the compound due to the presence of many confounding factors. These tests are also unable to be replicated in the exact manner.
In the future it will be prudent for the field of microplastic research in the aquatic environment to settle on a proper definition of microplastic and establish standardized techniques. Additionally, more studies should investigate the fate of microplastics in larger marine animals and in freshwater organisms. Additional data is needed to illustrate how HOCs, POPS, and additives interact with microplastics both outside and inside aquatic organisms for toxicological purposes. This will require the development of a highly thought out screening process that will allow multiple types of microplastics and possible toxicants to be evaluated simultaneously. It is impossible to test all combinations of microplastics with all HOCs, POPs, and additives in a lab. Thus, steps need to be taken to streamline the process.
Conclusion
[edit | edit source]The field of microplastic research in aquatic toxicology is a relatively new field. Microplastics are located worldwide in all types of aquatic environments. It has been well documented that microplastics can be ingested by both aquatic invertebrates and aquatic vertebrates. Additionally, microplastics degrade at a very slow rate through photodegradation and thermooxidative degradation. Although microplastics are not considered intrinsically toxic themselves, it is possible for chemicals to adsorb to particles and then leach off the microplastics once inside an organism. This is highly unlikely for HOCs and POPs, but more data is needed for additives. Finally, as with all new fields, this field has some shortcomings such as a lack of standardized definitions and techniques. It is expected that more solid definitions and protocols will emerge in the future.
Glossary
[edit | edit source]1. Additives: Compounds added to plastic while it is being synthesized.
2. Beach combing: Collecting, identifying, and quantifying all litter items on the shore of a specific coastline region.
3. Biodegradation: Process by which living organisms degrade microplastics or other chemicals.
4. Degradation: Chemical process by which the average molecular weight of a polymer is reduced.
5. Hydrolysis: Process by which water is used to degrade a compound.
6. Hydrophobic organic compounds (HOCs): Organic compounds that are more soluble in organic solutions than in water.
7. Leaching: Within the context of this chapter, a process by which a chemical leaves the microplastic and enters the aquatic environment.
8. Macroplastics : Plastics that are easy to see with the naked eye and are > 5 mm in diameter.
9. Marine observational sampling: Involves divers or onboard observers who record the size, location, and types of plastics in the water.
10. Marine trawls: Use of fine meshes or nets to collect microplastic particles or marine organisms.
11. Microplastic: Plastic particle that is < 5 mm in size but greater than 100 nm.
12. Nanoplastic: Plastics that are between 1-100 nm.
13. Persistent organic pollutants (POPs): Lipophilic chemicals that prefer to concentrate on hydrophobic regions of microplastics.
14. Photodegradation: Process by which light, usually sunlight, degrades a material.
15. Physical Adverse Response: Within the context of this chapter, a negative physiological response to a microplastic particle.
16. Plastics: Long chains of polymers that are synthesized from both organic and inorganic materials such as carbon, hydrogen, silicon, oxygen, and chloride; usually acquired from natural resources such as natural gas, oil and coal.
17. Primary microplastics: Plastics manufactured in the microplastic size range.
18. Secondary microplastics: Microplastic particles that have fragmented from macroplastics.
19. Thermal degradation: Degradation that occurs via high temperatures.
20. Thermooxidative degradation: Process by which particles slowly break down through oxidation in moderate temperatures.
21. Toxicant: Human-made chemicals that cause a deleterious or harmful response to an organism.
References
[edit | edit source]Avery-Gomm S, O’Hara PD, Kleine L, Bowes V, Wilson LK, Barry KL. 2012. Northern fulmars as biological monitors of trends of plastic pollution in the eastern North Pacific. Marine Pollution Bulletin. 64: 1776-1781.
Avery-Gomm S, Provencher JF, Morgan KH, Bertram DF. 2013. Plastic ingestion in marine-associated bird species form the eastern North Pacific. Marine Pollution Bulletin. 72: 275-259.
Andrady, A. 2011. Microplastics in the marine environment. Marine Pollution Bulletin. 62: 1596-1605.
Barnes DKA, Galgani F, Thompson RC, Barlaz M. 2009. Environmental accumulation and fragmentation of plastic debris in global. Philosophical Transactions of the Royal Society B. 364: 1985-1998.
Boerger CM, Lattin GL, Moore SL, Moore CJ. 2010. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Marine Pollution Bulletin. 60: 2275-2278.
Braid HE, Deeds J, Degrasse JL, Wilson JJ, Osborne J, Hanner RH. 2012. Preying on commercial fisheries and accumulating paralytic shellfish toxins: a dietary analysis of invasive Dosidicus gigas (Cephalopoda Ommastrephidae) stranded in Pacific Canada. Marine Biology. 159: 25-31.
Browne MA, Dissanayake A, Galloway TS, Lowe DM, Thompson RC. 2008. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis. Environmental Science & Technology. 42: 5026-5031.
Carpenter EJ, Anderson SJ, Harvey GR, Miklas HP, Peck BB. 1972. Polystyrene spherules in coastal waters. Science. 175: 749-750.
Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS. 2013. Microplastic ingestion by zooplankton. Environmental Science & Technology. 47: 6646-6655.
Cole M, Lindeque P, Halsband C, Galloway TS. 2011. Microplastics as contaminants in the marine environment: a review. Marine Pollution Bulletin. 62: 2588-2597.
Dantas DV, Barletts M, Costa MF. 2012. The seasonal and spatial patterns of ingestion of polyfilament nylon fragments by estuarine drums (Sciaenidae). Environmental Science and Pollution Research. 19: 600-606.
Davidson P, Asch RG. 2011. Plastic ingestion by mesopelagic fishes in the North Pacific Subtropical Gyre. Marine Ecology Progress Series. 432: 173-180.
Ed. Klaassen, C. 2013. Casarett and Doull’s Toxicology: The Basic Science of Poison.
Eriksson C, Burton H. 2003. Origins and biological accumulation of small plastic particles in fur seals from Macquarie Island. AMBIO: A Journal of the Human Environment. 32: 380-384.
Farrell P, Nelson K. 2012. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environmental Pollution. 177: 1-3.
Goldstein MC, Rosenberg M, Cheng L. 2012. Increased oceanic microplastic debris enhances oviposition in an endemic pelagic insect. Biology Letters. 8: 817-820.
Graham E, Thompson J. 2009. Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. Journal of Experimental Marine Biology and Ecology. 368: 22-29.
Gray H, Lattin G, Moore CJ. 2012. Incidence, mass and variety of plastics ingested by Laysan (Phoebastria immutabilis) and Black-footed Albatrosses (P. nigripes) recovered as by-catch in the North Pacific Ocean. Marine Pollution Bulletin. 64, 2190-2192.
Güven, O., Gökdağ, K., Jovanović, B. & Kıdeyş, A.E. 2017. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environmental Pollution 223, 286-294
Hoss DE, Settle LR. 1990. Ingestion of plastic by teleost fishes. In: Shomura RS, Godrey ML, editors. Proceedings of the Second International Conference on Marine Debris. 2-7 April 1989, Honolulu, Hawaii. US. Department of Commerce, pp 693-709. NOAA Tech. Memo. NMFS, NOAA-TM-NMFS-SWFC-154.
Ivar do Sul J, Costa MF. 2014. The present and future of microplastic pollution in the marine environment. Environmental Pollution. 185: 352-364.
Jovanović, B. 2017. Ingestion of microplastics by fish and its potential consequences from a physical perspective. Integrated Environmental Assessment and Managements. 13 (3): 510-515.
Koelmans AA. 2015. Modeling the role of microplastics in bioaccumulation of organic chemicals to marine aquatic organisms. A critical review. In: Bergmann M. Gutow L, Klages M, editors. Marine anthropogenic litter. Cham (CH): Springer International Publishing. p 309-324.
Koelmans AA, Bakir A, Burton GA, Janssen C. 2016. Microplastics as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environmental Science & Technology. 50: 3315-3326.
Koelmans AA, Besseling E, Shim WJ. 2015. Nanoplastics in the aquatic environment. Critical Review. In: Bergmann M, Gutow L, Klages M, editors. Marine anthropogenic litter. Cham (CH): Springer International Publishing. p 326-342.
Koelmans, A. A., Besseling, E., Wegner, A., & Foekema, E. M. (2013a). Plastic as a carrier of POPs to aquatic organisms: A model analysis. Environmental Science & Technology. 47, 7812–7820.
Koelmans, A. A., Besseling, E., Wegner, A., & Foekema, E. M. (2013b). Correction to plastic as a carrier of POPs to aquatic organisms: A model analysis. Environmental Science & Technology. 47, 8992–8993.
Koelmans, A. A., Besseling, E., & Foekema, E. M. (2014b). Leaching of plastic additives to marine organisms. Environmental Pollution. 187, 49–54.
Kühn S, van Franeker JA. 2012. Plastic ingestion by the northern fulmar (Fulmarus glacialis) in Iceland. Marine Pollution Bulletin. 64: 1252-1254.
Kwon JH, Chang S, Hong SH, Shim WJ. 2017. Microplastics as a vector of hydrophobic contaminants: importance of hydrophobic additives. Integrated Environmental Assessment and Management.t 13 (3): 494-499.
Lindborg VA, Ledbetter JF, Walat JM, Moffett C. 2012. Plastic consumption and diet of Glaucous-winged Gulls (Larus glaucescens). Marine Pollution Bulletin. 64: 2351-2356.
Lusher AL, McHugh M, Thompson RC. 2013. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Marine Pollution Bulletin. 67: 94-99.
Majer AP, Vedolin MC, Turra A. 2012. Plastic pellets as oviposition site and means of dispersal for the ocean-skater insect Halobates. Marine Pollution Bulletin. 64: 1143-1147.
Murray F, Cowie PR. 2011. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Marine Pollution Bulletin. 62: 1207-1217.
PlasticsEurope. 2018. Plastics – the facts 2018: an analysis of European plastics production, demand and waste data. https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics_the_facts_2018_AF_web.pdf
Possatto FE, Barletta M, Costa MF, Ivar do Sull, JA, Dantas DV. 2011. Plastic debris ingestion by marine catfish: an unexpected fisheries impact. Marine Pollution Bulletin. 62: 1098-1102.
Provencher JF, Gaston AJ, Mallory ML. 2009. Evidence for increased ingestion of plastics by northern fulmars (Fulmarus glacialis) in the Canadian Arctic. Marine Pollution Bulletin. 58: 1092-1095.
Provencher JF, Gaston AJ, Mallory ML, O’hara PD, Gilchrist HG. 2010. Ingested plastic in a diving seabird, the thick-billed murre (Uria lomvia), in the eastern Canadian Arctic. Marine Pollution Bulletin. 60: 1406-1411.
Ramos JAA, Barletta M, Coast MF. 2012. Ingestion of nylon threads by Gerreidae while using a tropical estuary as foraging grounds. Aquatic Biology. 17: 29-34.
Rodríguez A, Rodríguez B, Carrasco MN. 2012. High prevalence of parental delivery of plastic debris in Cory’s shearwaters (Calonectris diomedea). Marine Pollution Bulletin. 64: 2219-2223.
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AWG, McGonigle D, Russell, AE. 2004. Lost at sea: where is all the plastic? Science. 304: 838.
Tueten EL, Rowland SJ, Galloway TS, Thompson RC. 2007. Potential for microplastics to transport hydrophobicm contaminants. Environmental Science & Technology. 41 (22): 7759-7764.
Van Franeker JA, Bell PJ. 1988. Plastic ingestion by petrels breeding in Antarctica. Marine Pollution Bulletin. 19: 672-674.
Van Franeker JA, Blaize C, Danielsen J, Fairclough K, Gollan J, Guse N, Hansen PL, Huebeck M, Jensen JK, Le Guillou G, Olsen B, Olsen KO, Pedersen J, Stienen EWM, turner DM. 2011. Monitoring plastic ingestion by the northern fulmar Fulmarus glacialis in the North Sea. Environmental Pollution. 159: 2609-2615.
Van Noord JE. 2013. Diet of five species of the family Myctophidae caught off the Mariana Islands. Ichthyological Research. 60 (1): 89-92.
Van Sebille E, Wilcox, C, Lebreton L, Maximenko N, Hardesty BD, van Franeker JA, Eriksen M, Siegel D, Galgani F, Law KL. 2015. A global inventory of small floating plastic debris. Environmental Research Letters. 10.
Von Moos N, Burkhardt-Holm P. Köhle A. 2012. Uptake and effects of microplastics on cells and tissues of the blue mussel Mytilus edulis L. after and experimental exposures. Environmental Science & Technology. 46: 11327-11335.
Chapter Four: Analytical Methodology for Detecting & Monitoring Synthetic Organic Contaminants in the Aquatic Environment
Introduction
[edit | edit source]Synthetic Organic contaminants are man-made carbon-based chemicals which often enter aquatic environments through drainage from agricultural land and through discharge from industrial areas (1). The United States Environmental Protection Agency has put legal limits on the concentration that can be present in drinking water of 56 organic contaminates due to their potential adverse effects to humans (1). However, this does not stop these contaminants from entering aquatic ecosystems. Aquatic biomes encompass the largest portion of Earth’s surface (~75%) and are often divided into freshwater and saltwater biomes. Aquatic biomes are home to more than a million different plant and animal species and provide many ecosystem services. Examples of these services include fish harvest (food), wild plant and animal resources, water, recreation, flood control, carbon sequestration and storm protection which make aquatic ecosystems and their protection vital for human success (2). Therefore, we must address issues of pollution and work to understand the potential risk associated with it.



