Organic Chemistry/Organometallics
Organometallics is the branch of chemical science studying the chemistry of molecules that have direct carbon-metal bonds.

Main group organometallic chemistry
[edit | edit source]Alkali and alkaline earths organometallic
[edit | edit source]- Li, Na, K organyls
- Be organyls
- Mg organyls
Aluminium group
[edit | edit source]Silicon group
[edit | edit source]- Si organyls
- Ga organyls
Pb, Sb, Sn, Hg
[edit | edit source]Transition-metal organometallic chemistry
[edit | edit source]The organometallic chemistry of the transition elements is quite different from the main-group ones due to the availability for bonding of the n d orbitals with consequent ability for the central atom to change geometry and expand the octet.
Single σ-bonding
[edit | edit source]- M-alkyl
β-elimination
[edit | edit source]π-acceptor bonding
[edit | edit source]alkene complexes
[edit | edit source]CO complexes
[edit | edit source]- σ *→dσ
- dπ→π *
These interactions are synergicin increading the M-CO bond strength. In fact, the second interaction, as known as pi backbonding increases the available electron density on the CO.
The partial filling of the π* orbital leads to a weakened C-O triple bond, as showed from the stretching frequencies (in cm-1) of CO free and in M/CO complexes.
| free CO | 2143 |
| V(CO)6 | 1976 |
| Ni(CO)4 | 2057 |
| Cr(CO)6 | 2000 |
Arene complexes
[edit | edit source]Carbenes and carbynes compounds
[edit | edit source]Fischer carbenes
[edit | edit source]By treatment of a CO complex with a strong nucleophile

Schrock carbenes
[edit | edit source]Catalysis by organometallic compounds
[edit | edit source]Metathesis
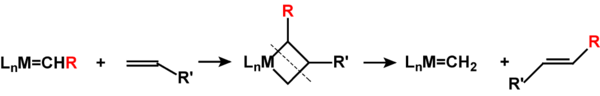
[edit | edit source]Richard Schrock(MIT, USA) and Robert Grubbs (CalTech, USA) received 2005 Nobel prize for their work on the subject. Metathesis is the exchange of the termination between two alkenes [1]
It occurs via the carbene species nowaday known as Schrock's carbenes
- ^ Grubbs, Olefin metathesis, Tetrahedron
Ziegler-Natta polymerisation
[edit | edit source]Ziegler-Natta catalyst Ziegler in the 40's worked on the oligomerisation of ethylene by aluminium alkyls via the reaction HAl-R + CH2=CH2 -> HAl-CH2CH2-R
Enantioselective hydrogenation
[edit | edit source]Wilkinson's
Hydroformylation
[edit | edit source]Hydroformylation Hydroformylation is the process that transforms an alkene into an aldehyde by reaction with CO.
The catalyst is a hydridocarbonyl complex, HCO(CO)5

Fischer-Tropsch synthesis
[edit | edit source]Fischer-Tropsch synthesis is the heterogeneously-catalysed formation of hydrocarbons (alkanes and alkenes) from CO and hydrogen (synthesis gas). It can be seen as the inverse of synthesis gas preparation (although this is usually from methane and lighter hydrocarbons). It is the heart of the gas-to-liquids processes developed commercially by big petrochemical firms in the 90's.
Organometallics in living systems
[edit | edit source]The only example of a biological molecule containing direct carbon-metal bonds is cobalamin, as known as vitamin B12