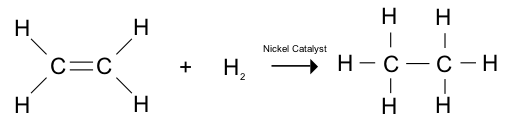
Organic Chemistry/Introduction to reactions/Hydrogenation
The result of hydrogenation reactions is to increase the number of hydrogens on a molecule. The recipient molecule is typically an alkene whose double bond is reduced by the addition of a hydrogen to each of the two carbons.
The mechanism of this reaction is usually through a catalyst, such as Nickel, Platinum (used in PtO2, otherwise known as Adams' Catalyst after Roger Adams) or Palladium (Pd) that is mixed onto an inert material like charcoal (sometimes referred to as Palladium on carbon). The reaction takes place not within a solution but actually specifically on the surface of the catalyst.
First the H2 gas is absorbed onto the catalyst surface.
The Alkene forms a complex with the catalyst surface due to the vacancy of the orbital on the metal interacting with the filled π orbital. Thus we have a structure that looks somewhat like this:
Once the complex is formed the hydrogen is able to apply itself to the double bond twice and then the final product is fully saturated and floats away from the catalyst, thus regenerating it. Hydrocarbons are of two types, aliphatic and aromatic. There are different methods to identify a hydrocarbon, if no of C atoms: 1 then Meth.