Molecular Cloning/westarn blot
The Western blot (alternatively, protein immunoblot) is a widely used analytical technique used to detect specific proteins in the given sample of tissue homogenate or extract.
Sample preparation
[edit | edit source]Samples may be taken from whole tissue or from cell culture. In most cases, solid tissues are first broken down mechanically using a blender (for larger sample volumes), using a homogenizer (smaller volumes), or by sonication. Cells may also be broken open by one of the above mechanical methods. However, bacteria, virus or environmental samples can be the source of protein and thus Western blotting is not restricted to cellular studies only. Assorted detergents, salts, and buffers may be employed to encourage lysis of cells and to solubilize proteins. Protease and phosphatase inhibitors are often added to prevent the digestion of the sample by its own enzymes. Tissue preparation is often done at cold temperatures to avoid protein denaturing and degradation. A combination of biochemical and mechanical techniques – including various types of filtration and centrifugation – can be used to separate different cell compartments and organelles.
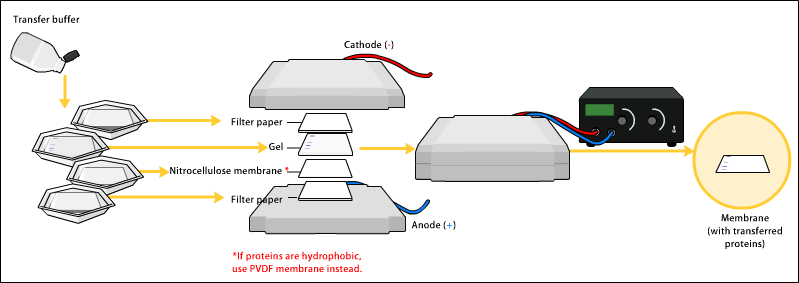
Transfer of protein from gel to membrane
[edit | edit source]In order to make the proteins accessible to antibody detection, they are moved from within the gel onto a membrane made of nitrocellulose or polyvinylidene difluoride (PVDF). The membrane is placed on top of the gel, and a stack of filter papers placed on top of that. The entire stack is placed in a buffer solution which moves up the paper by capillary action, bringing the proteins with it. Another method for transferring the proteins is called electroblotting and uses an electric current to pull proteins from the gel into the PVDF or nitrocellulose membrane. The protein move from within the gel onto the membrane while maintaining the organization they had within the gel. As a result of this "blotting" process, the proteins are exposed on a thin surface layer for detection (see below). Both varieties of membrane are chosen for their non-specific protein binding properties (i.e. binds all proteins equally well). Protein binding is based upon hydrophobic interactions, as well as charged interactions between the membrane and protein. Nitrocellulose membranes are cheaper than PVDF, but are far more fragile and do not stand up well to repeated probings. The uniformity and overall effectiveness of transfer of protein from the gel to the membrane can be checked by staining the membrane with Coomassie Brilliant Blue or Ponceau S dyes. Ponceau S is the more common of the two, due to Ponceau S's higher sensitivity and its water solubility makes it easier to subsequently destain and probe the membrane as described below.
Detection of protein band
[edit | edit source]During the detection process the membrane is "probed" for the protein of interest with a modified antibody which is linked to a reporter enzyme, which when exposed to an appropriate substrate drives a colourimetric reaction and produces a colour. For a variety of reasons, this traditionally takes place in a two-step process, although there are now one-step detection methods available for certain applications.
Primary antibody

Primary antibodies are generated when a host species or immune cell culture is exposed to the protein of interest (or a part thereof). Normally, this is part of the immune response, whereas here they are harvested and used as sensitive and specific detection tools that bind the protein directly. After blocking, a dilute solution of primary antibody (generally between 0.5 and 5 micrograms/mL) is incubated with the membrane under gentle agitation. Typically, the solution is composed of buffered saline solution with a small percentage of detergent, and sometimes with powdered milk or BSA. The antibody solution and the membrane can be sealed and incubated together for anywhere from 30 minutes to overnight. It can also be incubated at different temperatures, with warmer temperatures being associated with more binding, both specific (to the target protein, the "signal") and non-specific ("noise").
Secondary antibody After rinsing the membrane to remove unbound primary antibody, the membrane is exposed to another antibody, directed at a species-specific portion of the primary antibody. Antibodies come from animal sources (or animal sourced hybridoma cultures); an anti-mouse secondary will bind to almost any mouse-sourced primary antibody, which allows some cost savings by allowing an entire lab to share a single source of mass-produced antibody, and provides far more consistent results. This is known as a secondary antibody, and due to its targeting properties, tends to be referred to as "anti-mouse," "anti-goat," etc. The secondary antibody is usually linked to biotin or to a reporter enzyme such as alkaline phosphatase or horseradish peroxidase. This means that several secondary antibodies will bind to one primary antibody and enhance the signal. Most commonly, a horseradish peroxidase-linked secondary is used to cleave a chemiluminescent agent, and the reaction product produces luminescence in proportion to the amount of protein. A sensitive sheet of photographic film is placed against the membrane, and exposure to the light from the reaction creates an image of the antibodies bound to the blot. A cheaper but less sensitive approach utilizes a 4-chloronaphthol stain with 1% hydrogen peroxide; reaction of peroxide radicals with 4-chloronaphthol produces a dark brown stain that can be photographed without using specialized photographic film.
Analysis of blot
[edit | edit source]After the unbound probes are washed away, the Western blot is ready for detection of the probes that are labeled and bound to the protein of interest. In practical terms, not all Westerns reveal protein only at one band in a membrane. Size approximations are taken by comparing the stained bands to that of the marker or ladder loaded during electrophoresis. The process is repeated for a structural protein, such as actin or tubulin, that should not change between samples. The amount of target protein is indexed to the structural protein to control between groups. This practice ensures correction for the amount of total protein on the membrane in case of errors or incomplete transfers.
Colorimetric detection The colorimetric detection method depends on incubation of the Western blot with a substrate that reacts with the reporter enzyme (such as peroxidase) that is bound to the secondary antibody. This converts the soluble dye into an insoluble form of a different color that precipitates next to the enzyme and thereby stains the membrane. Development of the blot is then stopped by washing away the soluble dye. Protein levels are evaluated through densitometry (how intense the stain is) or spectrophotometry.
Chemiluminescent detection Chemiluminescent detection methods depend on incubation of the Western blot with a substrate that will luminesce when exposed to the reporter on the secondary antibody. The light is then detected by photographic film, and more recently by CCD cameras which capture a digital image of the Western blot. The image is analysed by densitometry, which evaluates the relative amount of protein staining and quantifies the results in terms of optical density. Newer software allows further data analysis such as molecular weight analysis if appropriate standards are used.
Fluorescent detection The fluorescently labeled probe is excited by light and the emission of the excitation is then detected by a photosensor such as CCD camera equipped with appropriate emission filters which captures a digital image of the Western blot and allows further data analysis such as molecular weight analysis and a quantitative Western blot analysis. Fluorescence is considered to be among the most sensitive detection methods for blotting analysis.
Secondary probing One major difference between nitrocellulose and PVDF membranes relates to the ability of each to support "stripping" antibodies off and reusing the membrane for subsequent antibody probes. While there are well-established protocols available for stripping nitrocellulose membranes, the sturdier PVDF allows for easier stripping, and for more reuse before background noise limits experiments. Another difference is that, unlike nitrocellulose, PVDF must be soaked in 95% ethanol, isopropanol or methanol before use. PVDF membranes also tend to be thicker and more resistant to damage during use.