Historical Geology/Radioactive decay
As a prelude to the articles on radiometric dating, it is desirable that the reader should know something about the mechanisms of radioactive decay. This article provides a fairly non-technical explanation of what it is and how it works.
Isotopes
[edit | edit source]The reader should recall from high school that the nucleus of an atom consists of protons (positively charged particles) and neutrons (uncharged particles which have almost exactly the same mass as protons). The nucleus is surrounded by a cloud of electrons, negatively charged particles having negligible weight. The number of electrons is equal to the number of protons.
The number of protons in an atom is its atomic number, and the sum of the protons and the neutrons gives its atomic weight.
The chemical properties of an atom are determined by the behavior of its electrons, and so are in effect determined by its atomic number. Hence in chemistry atoms are classified into elements according to their atomic number: an element such as carbon, for example, is defined by having an atomic number of 6. However, two atoms can have the same atomic number and different atomic weights. So, for example, 12C (carbon-12) has six protons and six neutrons, whereas 14C (carbon-14) has six protons and eight neutrons. They are both carbon, and they both behave chemically as though they are carbon, but they have a different atomic weight. So they are said to be the same element, namely carbon, but to be different isotopes of carbon. An isotope is defined by its atomic number and its atomic weight.
In the example in the previous paragraph, we have shown the notation used for isotopes. An atom with six protons and eight neutrons is written as 14C. The fact that it has six protons is revealed by the "C", which is the chemical symbol for carbon; by definition, all carbon atoms have six protons. The fact that it has eight neutrons is revealed by the little "14" written above and to the left of the "C": this is the atomic weight of the isotope, and so since the atomic weight is the number of protons plus the number of neutrons, and since all carbon atoms have six protons, this tells us that this isotope of carbon must have eight neutrons.
Radioactive decay
[edit | edit source]Radioactive decay may be defined as any spontaneous event which changes the state of the nucleus, emitting energy from the nucleus in the process. With the exception of gamma decay, which need not concern us here, this will involve changing the number of protons, or neutrons, or both, and so also changing the atomic number, the atomic weight, or both.
There are a number of mechanisms by which decay may take place. For our purposes, the most important are:
- Alpha decay. In this form of decay, the nucleus ejects an alpha particle consisting of two neutrons and two protons, reducing the atomic number by two and the atomic weight by four.
- Beta minus decay. In this form of decay, one of the neutrons in the atom is converted to a proton by the atom emitting an electron. Hence, the atomic number goes up by one, while the atomic weight stays the same.
- Beta plus decay. In this form of decay, a proton is converted into a neutron by the emission of a positron (a particle like an electron only positively charged) the result being that the atomic number goes down by one while the atomic weight stays the same.
- Electron capture. In this form of decay, one of the atom's own electrons combines with one of its protons, converting the proton into a neutron. This reduces the atomic number by one while leaving the atomic weight the same.
When decay takes place, the original atom is called the parent atom, and the new atom produced by decay is called the daughter atom. Those isotopes which are produced by radioactive decay are said to be radiogenic. Not all isotopes undergo decay: those that do are called unstable isotopes (or radioactive isotopes) and conversely those that don't are called stable. So for example 12C is stable and will go on being 12C forever; by contrast 14C is unstable and has a tendency to decay into 14N (nitrogen-14). As we can see from this example, it is perfectly possible for different isotopes of the same element to differ in their stability.
The reader should note that when a parent atom decays to a daughter atom, the daughter is not necessarily stable; sometimes the daughter will undergo further decay. Such a situation is described as a decay chain.
Statistics of radioactive decay
[edit | edit source]It is important to understand how and why radioactive decay takes place. According to physicists, radioactive decay occurs at random: an atom of (for example) 22Na (sodium-22) will undergo beta decay and produce an atom of 22Ne (neon-22) just because its number has come up.
The age of the atom has nothing to do with it. Consider, by analogy, a man playing Russian Roulette with a six-shooter. Every single time he plays, he has a one-in-six chance of dying, and this is true no matter how long he's been playing. The same is true of radioactive decay. If we have an atom of 22Na, then no matter how old it is, it has a 50% chance of decaying in the next 2.6 years; and if it survives that period of time, then it has a 50% chance of decaying in the next 2.6 years; and so on.
Consider what this means if we have a large sample of 22Na. Because the sample is large, its behavior will closely approximate our statistical expectations for it: so after 2.6 years 50% of the atoms will have decayed to 22Ne; after a further 2.6 years 50% of the remaining 22Na will have decayed (leaving only 25% of the original 22Na); after a further 2.6 years 50% of those 22Na atoms will have decayed, leaving 12.5% of the original sample; and so forth.
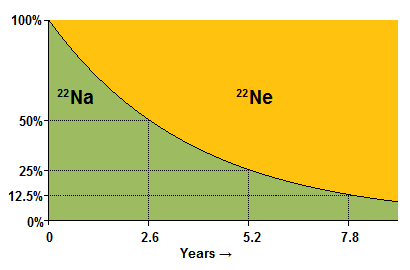
This figure of 2.6 years is known as the half-life of 22Na: that is, the time over which an atom of 22Na has a 50% chance of decaying; or, equivalently the time over which our statistical expectation is that 50% of the sample will have decayed.
Such situations are mathematically well-understood, and can be represented by the equation for exponential decay:
- N(t) = N0 × 2-t/2.6
where N0 is the original quantity of 22Na, 2.6 is the half-life in years, t is the time elapsed as measured in years, and N(t) is the quantity of 22Na left in the sample at time t.
The situation can be represented by the graph to the right.
For ease of exposition, we have used 22Na consistently as an example, but the same rules apply to all radioactive isotopes, the only difference is that the half-lives of different isotopes will be different: for example 107Pd (palladium-107) has a half-life of 6.5 million years.
People are sometimes startled by such a statement: how, they ask, is it possible to say this when we haven't been watching a sample of 107Pd for 6.5 million years to check that this is how long it takes for half of it to decay?
However, this is not really a problem. After all, by analogy, it is not necessary for a police officer to observe your car for an hour to report that you were traveling at 72 kilometers per hour. It is sufficient to observe you traveling at 20 meters in a single second, and then do the math.
In the same way, if we spend just a single year observing a sample of 107Pd and see that over that time only 0.0000106638% of the sample decays, then we can write:
- 100 - 0.0000106638 = 100 × 2-1/h
and then solving the equation for h will give us the half-life in years.
It is obvious from this example that the exactness of our knowledge of the half-life will depend on the exactness with which we can measure the initial size of the sample and the rate at which it decays.
Invariance of the half-life
[edit | edit source]Each unstable isotope, then, has its own characteristic half-life. What is more, for each isotope, this half-life is constant: it is a property of the isotope, and virtually unaffected by external circumstances.
How do we know this? In the first place, it should be true in principle: it can be deduced from the underlying laws of quantum mechanics. In the second place it is confirmed by actual observation: shortly after the discovery of radioactive decay, scientists began trying to change the decay rate by subjecting unstable isotopes to heat, pressure, magnetism, and so forth, with negative results.
Some small variations have been observed in certain isotopes depending on which other chemicals they form molecular bonds with. An example is 7Be (beryllium-7) in which the decay rate varies by about 1% according to the chemical environment it's in. There are theoretical reasons why 7Be ought to be particularly susceptible to such effects: it is a very small atom, and it decays by electron capture. Even so, the variation is fairly small. For the same reasons, even smaller variations have been achieved by putting 7Be under intense pressure.
We should also mention the peculiar isotope 187Re (rhenium-187). Because the energy emitted from the nucleus when it decays is so very small, it is possible to change its half-life by ionizing it. If it is completely stripped of all its electrons, its half-life falls from 43 billion years to 33 years! However, as this will happen nowhere on Earth outside a physics laboratory, we can take its half-life to be 43 billion years for all geological purposes.
So to really affect the half-life of an isotope one needs to resort to highly artificial methods — such as dropping it into the core of a nuclear reactor. In nature, and in particular in rocks, there are sound theoretical and observational reasons to conclude that unstable isotopes will have constant or very nearly constant half-lives and so will undergo decay, and will have undergone decay, in a regular and predictable manner.
Radiometric dating
[edit | edit source]The astute reader will probably have figured out where this is going. In the decay of unstable isotopes, we have a set of natural processes each of which goes at an utterly predictable rate.
Naively speaking, the idea behind radiometric dating goes something like this. If we have a rock and we know the original quantity of some radioactive isotope that the rock contained when it was first formed, and if we know its half-life, and if we can accurately measure the quantity of the isotope in the rock at the present time, then we can figure out how old the rock is. Or alternatively if we knew the original quantity and the present quantity of the daughter isotope of the radioactive isotope, then again we could figure out the age of the rock. Such a technique of absolute dating is known as radiometric dating.
Only how can we know the original composition of the rock? The answer to this question varies depending on which radioactive isotope we're talking about; so at this point it is time to stop talking in generalities and instead look at particular methods of radiometric dating in detail. These will be the subject of the next few articles.
