General Chemistry/Redox Reactions/Electrochemistry
Redox Reactions (review)
[edit | edit source]Redox (shorthand for reduction/oxidation reaction) describes all chemical reactions in which atoms have their oxidation number (oxidation state) changed.
This can be either a simple redox process such as the oxidation of carbon to yield carbon dioxide, or the reduction of carbon by hydrogen to yield methane (CH4), or it can be a complex process such as the oxidation of sugar in the human body through a series of very complex electron transfer processes.
The term redox comes from the two concepts of reduction and oxidation. It can be explained in simple terms:
- Oxidation describes the loss of electrons by a molecule, atom, or ion
- Reduction describes the gain of electrons by a molecule, atom, or ion
However, these descriptions (though sufficient for many purposes) are not truly correct. Oxidation and reduction properly refer to a change in oxidation number—the actual transfer of electrons may never occur. Thus, oxidation is better defined as an increase in oxidation number, and reduction as a decrease in oxidation number. In practice, the transfer of electrons will always cause a change in oxidation number, but there are many reactions which are classed as "redox" even though no electron transfer occurs noway (such as those involving covalent bonds).
Electrochemistry
[edit | edit source]Electrochemistry is a branch of chemistry that deals with the flow of electricity by chemical reactions. The electrons in a balanced half-reaction show the direct relationship between electricity and the specific redox reaction. Electrochemical reactions are either spontaneous, or nonspontaneous. A spontaneous redox reaction generates a voltage itself. A nonspontaneous redox reaction occurs when an external voltage is applied. The reactions that occur in an electric battery are electrochemical reactions.
- Three components of an electrochemical reaction
- A solution where redox reactions may occur (solutions are substances dissolved in liquid, usually water)
- A conductor for electrons to be transferred (such as a metal wire)
- A conductor for ions to be transferred (usually a salt bridge) e.g. filter paper dipped in a salt solution.
Electrolysis
[edit | edit source]An electrolysis experiment forces a nonspontaneous chemical reaction to occur. This is achieved when two electrodes are submersed in an electrically conductive solution, and the electrical voltage applied to the two electrodes is increased until electrons flow. The electrode receiving the electrons, or where the reduction reactions occur, is called the cathode. The electrode which supplies the electrons, or where the oxidation reactions occur, is called the anode.
A molten salt is an example of something that may be electrolyzed because salts are composed of ions. When the salt is in its solid state, the ions are not able to freely move. However, when the salt is heated enough until it melts (making it a molten salt), the ions are free to move. This mobility of the ions in the molten salt makes the salt electrically conductive. In the electrolysis of a molten salt, for example melted , the cation of the salt (in this case ) will be reduced at the cathode, and the anion of the salt (in this case ) will be oxidized at the anode:
- Cathode reaction: Na+ + e- → Na
- Anode reaction: 2Cl → Cl2 + 2e-
Aqueous solutions of salts can be electrolyzed as well because they are also electrically conductive. In aqueous solutions, there is an additional reaction possible at each the cathode and the anode:
- Cathode: 2H2O + 2e- → H2 + 2OH- (reduction of water)
- Anode: 2H2O → 4H+ + O2 + 4e- (oxidation of water)
With the addition of these two reactions, there are now two possible reactions at each electrode. At the cathode, either the reduction of the cation or the reduction of water will occur. At the anode, either the oxidation of the anion or the oxidation of water will occur. The following rules determine which reaction takes place at each electrode:
- Cathode: If the cation is a very active metal, water will be reduced. Very active metals include Li, Na, K, Rb, Cs, Ca, Sr, and Ba. If the cation is an active or inactive metal, the cation will be reduced.
- Anode: If the anion is a polyatomic ion, water will generally be oxidized. Specifically, sulfate, perchlorate, and nitrate ions are not oxidized; water will oxidize instead. Chloride, bromide, and iodide ions will be oxidized. If the anion in one salt is oxidized in an aqueous electrolysis, that same anion will also be oxidized in any other salt.
Galvanic Cells
[edit | edit source]The energy of a spontaneous redox reaction is captured using a galvanic cell. The following parts are necessary to make a galvanic cell:
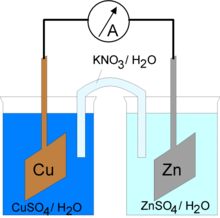
- Two half cells
- Two electrodes
- One electrically conductive wire
- One salt bridge
- One device, usually an ammeter or a voltmeter
A galvanic cell is constructed as shown in the image to the right. The two half-reactions are separated into two half cells. All of the reactants in the oxidation half-reaction are placed in one half cell (the anode), and all the reactants of the reduction half-reaction are placed in the other half cell (the cathode). If the half-reaction contains a metal, the metal serves as the electrode for that half cell. Otherwise, an inert electrode made of platinum, silver, or gold is used. The electrodes are connected with a wire which allows the flow of electrons. The electrons always flow from the anode to the cathode. The half cells are connected by a salt bridge which allows the ions in the solution to move from one half cell to the other, so that the reaction can continue. Since the overall reaction is spontaneous, the flow of electrons will move spontaneously through the outer circuitry from which the energy can be extirpated. The energy harnessed is useful because it can be used to do work. For example, if an electrical component such as a light bulb is attached to the wire, it will receive power from the flowing electrons.
Consistent results from a galvanic cell are dependent on three variables: pressure, temperature, and concentration. Thus, chemists defined a standard state for galvanic cells. The standard state for the galvanic cell is a pressure of 1.00 atmospheric pressure (atm) for all gases, a temperature of 298 kelvin (K) and concentrations of 1.00 molarity (M) for all soluble compounds, liquids, and solids.
Voltage
[edit | edit source]Voltage is a measure of spontaneity of redox reactions, and it can be measured by a voltmeter. If the voltage of a reaction is positive, the reaction occurs spontaneously, but when negative, it does not occur spontaneously.
To compute the voltage of a redox equation, split the equation into its oxidation component and reduction component. Then, look up the voltages of each component on a standard electrode potential table. This table will list the voltage for the reduction equation. The oxidation reaction's voltage is negative of the corresponding reduction equation's voltage. To find the equation's voltage, add the standard voltages for each half reaction.
Further reading
[edit | edit source]For those interested in the finer details of redox reactions, consider these books:



