General Chemistry/Properties of Matter/Classification of Matter
Matter can be classified by its state.
- Solids have a set volume and shape.The inter molecular force of attraction for solid matter is very strong.
- Liquids have a set volume, but change shape. The inter molecular force of attraction for liquid matter is weaker than solid matter.
- Gases have neither definite volume nor shape. The inter molecular force of attraction for gaseous matter is negligible.
- Plasma which are usually gaseous state of matter in which a part or all of the atoms or molecules are dissociated to form ions.
- Einstein-Bose Condensate (EBC) is a theoretical state of matter. The intermolecular force of attraction for EBC is so strong that the molecules cannot move whatsoever.
Matter can also be classified by its chemical composition.
- An element is a pure substance made up of atoms with the same number of protons. As of 2011, 118 elements have been observed, 92 of which occur naturally. Carbon (C), Oxygen (O), Hydrogen (H) are examples of elements. The periodic table is a tabular representation of the known elements.
- A compound consists of two or more chemical elements that are chemically bonded together. Water (H2O) and table sugar (C12H22O11) are examples of chemical compounds. The ratio of the elements in a compound is always the same. For example in water, the number of H atoms is always twice the number of O atoms.
- A mixture consists of two or more substances (element or compound) mixed together without any chemical bond. Salad is a good example. A mixture can be separated into its individual components by mechanical means.
Types of Mixtures
[edit | edit source]There are many kinds of mixtures. They are classified by the behavior of the phases, or substances that have been mixed.
Homogeneous Mixtures
[edit | edit source]
A homogeneous mixture is uniform, which means that any given sample of the mixture will have the same composition. Air, sea water, and carbonation dissolved in soda are all examples of homogeneous mixtures, or solutions. No matter what sample you take from the mixture, it will always be composed of the same combination of phases. Chocolate chip ice cream is not homogeneous—one spoonful taken might have two chips, and then another spoonful might have several chips.
An example for a homogeneous mixture is a solution. The substance that gets dissolved is the solute. The substance that does the dissolving is the solvent. Together they make a solution. If you stir a spoonful of salt into a glass of water, salt is the solute that gets dissolved. Water is the solvent. The salty water is now a solution, or homogeneous mixture, of salt and water.
When different gases are mixed, they always form a solution. The gas molecules quickly spread out into a uniform composition.
Heterogeneous Mixtures
[edit | edit source]A heterogeneous mixture is not uniform. Different samples may have different compositions, like the example of chocolate chip ice cream. Concrete, soil, blood, and salad are all examples of heterogeneous mixtures.

Suspensions
[edit | edit source]When sand gets kicked up in a pond, it clouds the water. It has a greater mass than water hence it sinks to the bottom and settles down, and is no longer mixed into the water. This is an example of a suspension. Suspensions are heterogeneous mixtures that will eventually settle. They are usually, but not necessarily, composed of phases in different states of matter. Italian salad dressing has three phases: the water, the oil, and the small pieces of seasoning. The seasonings are solids that will sink to the bottom, and the oil and water are liquids that will separate.
Colloids
[edit | edit source]
What exactly is toothpaste? We can't exactly classify it by its state of matter. It has a definite shape and volume, like a solid. But then you squeeze the tube, and it flows almost like a liquid. And then there's jelly, shaving cream, smoke, dough, and Silly Putty...
These are examples of colloids. A colloid is a heterogeneous mixture of two substances of different phases. Shaving cream and other foams are gas dispersed in liquid. Jello, toothpaste, and other gels are liquid dispersed in solid. Dough is a solid dispersed in a liquid. Smoke is a solid dispersed in a gas.
Colloids differ from suspensions in that they will not settle. |
Colloids consist of two phases: a dispersed phase inside of a continuous medium.
The Tyndall Effect
[edit | edit source]The Tyndall effect distinguishes colloids from solutions. In a solution, the particles are so fine that they will not scatter light. This is not true for a colloid. If you shine light through a solution, the beam of light will not be visible. It will be visible in a colloid. For instance, if you have ever played with a laser pointer, you have seen the Tyndall effect. You cannot see the laser beam in air (a solution), but if you shine it into a mist, the beam is visible. Clouds look white (or gray), as opposed to blue, because of the Tyndall effect - the light is scattered by the small droplets of suspended water.
Methods for Separating Mixtures
[edit | edit source]
Because there is no chemical bonding in a mixture, the phases can be separated by mechanical means. In a heterogeneous mixture like a salad, the pieces can easily be picked out and separated. It is as simple as sifting through the salad and picking out all the tomatoes and radishes, for example. However, many mixtures contain particles that are too small, liquids, or too many particles to be separated manually. We must use more sophisticated methods to separate the mixture.
Filtration
[edit | edit source]Imagine you have a sandbox, but there are bits of broken glass in it. All you would need is some sort of filter. The sand particles are much smaller than the glass chips, so a mesh filter would let sand pass but stop the glass. Filtration is used in all sorts of purification methods. Some filters, like dialysis tubing, are such fine filters that water can pass, but dissolved glucose cannot.
Filtration works with particles that are significantly different in size, like sand and rock, or water and glucose. |
Distillation
[edit | edit source]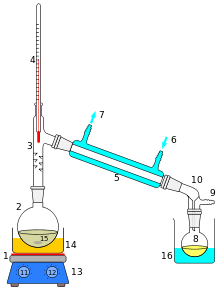
If you were given a glass of saltwater, could you drink it? Sure, if you distill it first. Distillation is the boiling of a mixture to separate its phases. Salt is a solid at room temperature, and water is a liquid. Water will boil far before salt even begins to melt. So separating the two is as simple as boiling the water until all that remains is the solid salt. If desired, the water vapor can be collected, condensed, and used as a source of pure water.
Distillation can also be used if two liquids are mixed but have different boiling points. Separation of several liquids with similar boiling points can be achieved using fractionation.
Centrifugation and Sedimentation
[edit | edit source]
These processes rely on differences in density. In a medical lab, blood often goes into a centrifuge. A centrifuge is a machine that spins a sample at fairly high rates of speed. Red blood cells are much denser than the watery substance (called plasma, but it's not the plasma state of matter) that makes up blood. As a result of the spinning, the denser phases move outward and the less dense phases move inward, towards the axis of rotation. Then, the red blood cells can be separated from the plasma.
Sedimentation is similar, but it happens when particles of different densities have settled within a liquid. If a jar of muddy water is left to settle, the heaviest particles sink to the bottom first. The lightest particles sink last and form a layer on top the heavier particles. You may have seen this effect in a bottle of salad dressing. The seasonings sink to the bottom, the water forms a lower layer, and the oil forms an upper layer. The separate phases can be skimmed out. To return it to a mixture, simply shake it up to disturb the layers.
Unique Properties
[edit | edit source]
The differences in substances' properties can be exploited to allow separation. Consider these examples:
- A mixture of sand and iron filings can be separated by magnet.
- Salt and sand can be separated by solution (sand will not dissolve in water, salt will)
- Helium can be separated from a mixture with hydrogen by combustion (this is a very dangerous operation, since hydrogen in the presence of oxygen is highly explosive). Hydrogen is flammable, but helium is not.
Other methods
[edit | edit source]There are countless other ways to separate mixtures. For instance, gel electrophoresis is used to separate different sized pieces of DNA. They are placed into gel, and an electric current is applied. The smaller pieces move faster and separate from the larger pieces.
Chromatography separates phases dissolved in liquid. If you want to see an example, take a strip of paper and draw a dot on it with a colored marker. Dip the strip into water, and wait a while. You should see the ink separate into different colors as they spread out from the dot.