General Chemistry/Introduction to Quantum Theory
Introduction to Quantum Mechanics
[edit | edit source]In the late 19th century, many physicists believed that they had made great progress in physics, and there wasn't much more that needed to be discovered. The classical physics at the time was widely accepted in the scientific community. However, by the early 20th century, physicists discovered that the laws of classical mechanics break down in the atomic world, and experiments such as the photoelectric effect completely contradict the laws of classical physics. As a result of these crises, physicists began to construct new laws of physics which would apply to the atomic world; these theories would be collectively known as quantum mechanics. Quantum mechanics, in some ways, completely changed the way physicists view the universe, and it also marked the end of the idea of a clockwork universe (the idea that the universe was predictable).
Electromagnetic Radiation
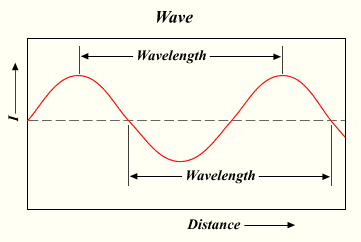
[edit | edit source]Electromagnetic radiation (ER) is a form of energy that sometimes acts like a wave, and other times acts like a particle. Visible light is a well-known example. All forms of ER have two inversely proportional properties: wavelength and frequency. Wavelength is the distance from one wave peak to the next, which can be measured in meters. Frequency is the number of wave peaks observed in a given point during a second. The unit for frequency is hertz.
Since wavelength and frequency are inversely related, their product (multiplication) always equals a constant — specifically, 3.0 x 108 m/sec represented by the letter c, which is better known as the speed of light. This relationship is written mathematically as , with the greek letter λ (lambda) representing wavelength and the letter representing frequency.
The wavelength and frequency of any specific occurrence of ER determine its position on the electromagnetic spectrum.

As you can see, visible light is only a tiny fraction of the spectrum.
The energy of a single particle of an electromagnetic wave (called a photon) is given by , where is Plank's constant and is the frequency. Energy is directly proportional to frequency — doubling the frequency will double the energy.
The Discovery of the Quantum
[edit | edit source]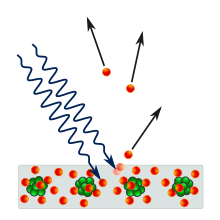
So far we have only discussed the wave characteristics of energy. However, the wave model cannot account for something known as the photoelectric effect. This effect is observed when light focused on certain metals apparently causes electrons to be emitted. (Photoelectric or solar panels work on this principle.)
For each metal it was found that there is a minimum frequency of electromagnetic radiation that is needed to be shone on it in order for it to emit electrons. This conflicted with the earlier thought that the energy of light was linked only to its intensity. Under that theory, the effect of light should be cumulative - dim light should add up, little by little, until it causes electrons to be emitted. Instead, there is a clear-cut minimum of the frequency of light that triggers the electron emissions.
The implication of this is that the energy of light is tied to frequency, and furthermore that it is quantized, meaning that it carries "packets" of energy in discrete amounts. These packets are more commonly referred to as photons. This observation led to the discovery of the minimum amount of energy that could be gained or lost by an atom. Max Planck named this minimum amount the quantum, plural "quanta", meaning "how much". One photon of light carries exactly one quantum of energy.