General Chemistry/Chemistries of Various Elements/Group 14
The Carbon Family
[edit | edit source]Group 14 (IVA) consists of carbon, silicon, germanium, tin, and lead. Carbon is a non-metal, silicon and germanium are metalloids, and tin and lead are metals.
With 4 valence shell electrons, elements of the carbon family tend to form covalent compounds. With increasing mass and atomic radius these elements become increasingly metallic and have lower melting and boiling points.
Group 14 elements form gaseous hydrogen compounds with difficulty. These are either unstable or combustible. All but lead form oxides, sulfides, and halides in the +4 oxidation state. The +4 oxidation state predominates in carbon, silicon, and germanium; the +2 and +4 oxidation states both appear in tin, and the +2 oxidation state prevails in lead. Halides in the +4 state form for all of these elements, and they are covalent.
Carbon compounds are much more covalent than analogous compounds of silicon, germanium, tin, or lead. Even more significantly, carbon forms double and even triple bonds with itself or other elements, forming compounds that the heavier elements of this group cannot form like acetylene (C2H2). Silicon and the heavier elements of this group can form only single bonds.
Thus carbon dioxide CO2 is a gas at normal temperatures because the double bonds between carbon and oxygen create single molecules, but silicon dioxide SiO2 forms a hard rock known as quartz because it is a covalent network solid. Each silicon atom bonds to four different oxygen atoms with single bonds, and each oxygen atom bonds with two silicon atoms. Similar properties apply to the oxides of germanium, tin, and lead. Carbon dioxide dissolves in water to form carbonic acid, a weak acid that reacts with bases to form carbonates; oxides of the other elements of this group are practically nonreactive in water.
Because of its unparalleled importance in chemistry, carbon is the main focus of our study of the Group 14 elements. |
Carbon
[edit | edit source]Carbon is a very important element. It is abundant in the earth and atmosphere, and it is found in the substances that make all living things. Carbon has many properties that make it different from other elements, so it deserves thorough study.
Allotropes
[edit | edit source]
Allotropes are different forms of a pure element. Carbon has several allotropes, three of which are common.
- Amorphous carbon is coal and soot. The carbon molecules are covalently bonded, but there is no order or arrangement.
- Graphite occurs when carbon forms flat covalent networks. These flat "sheets" are not bonded to each other, making them free to slide past each other. Graphite composes the "lead" in pencils.
- Diamond occurs when carbon forms a three-dimensional covalent network. Diamonds are much different from graphite and amorphous carbon. They are transparent, brilliant-looking, and incredibly hard. Diamond only forms at heat and intense pressures.
There are also several rare and exotic allotropes of carbon, including:
- Buckeyballs, or fullerenes, are spherical shaped balls of carbon. Covalent bonds join the carbon atoms into a soccer ball pattern that looks much like the geodesic domes of Buckminster Fuller. The most common buckeyball has the molecular formula C60. Buckeyballs are large enough for a small atom to get trapped inside.
- Lonsdaleite forms upon meteorite impact with the Earth.
- Carbon nanotubes are incredibly small but rigid tubes made of carbon. They are created in labs and are a subject of research.
Keep in mind that allotropes are composed of only one element. In this case, these allotropes contain only carbon atoms and no other elements.
Inorganic Compounds
[edit | edit source]Although carbon is known mainly for its organic compounds, it does form many important inorganic compounds.
Oxides
[edit | edit source]Oxides of carbon contain only carbon atoms and oxygen atoms. There are two oxides that occur commonly:
- Carbon monoxide (CO): a poisonous gas released when carbon-based fuels burn in limited oxygen.
- Carbon dioxide (CO2): found naturally in the air, but too much is considered pollution. Animals exhale carbon dioxide, and plants absorb it. It is slightly acidic. Carbon dioxide, when solid, is "dry ice".
There is other oxides that could form, but they are unstable or unnatural:

- Carbon suboxide (C3O2): Consists of double bonds, with oxygens at the ends. O = C = C = C = O. It breaks apart into carbon dioxide and dicarbon monoxide.
- Dicarbon monoxide (C2O): Very reactive. Contains only double bonds, but the end carbon has a non-bonding pair.
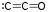
- Carbon trioxide (CO3): Exists in three different shapes, very unstable
Compounds Derived from Oxides
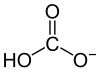
[edit | edit source]Carbonic acid forms when carbon dioxide is dissolved in water. It is given by the reaction:

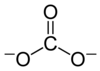
Carbonate and bicarbonate are two ions that carbon forms.
Their formulas are CO32- and HCO3-, respectively.
Ionic Compounds
[edit | edit source]Along with the oxyanions (carbonate and bicarbonate), carbon can form several other ions.

- Cyanide (CN-)
- Cyanate (OCN-)
- Thiocyante (SCN-)
- Carbides (C22- and C34-)
Hydrogen cyanide is an extremely-dangerous gas which causes death from interference with cellular metabolism. It has been used in executions and mass murder, most infamously in the Holocaust. |
Alloys
[edit | edit source]Carbon is used in some alloys, mixtures of metals. If a small amount (between 0.2% and 2.1% by weight) of carbon is mixed into iron, the result is steel.
Organic Compounds
[edit | edit source]
Compounds containing carbon (except for the above inorganic compounds) are considered organic. They were once thought to be produced only by living things, but they have since been created in laboratories. Most organic compounds contain hydrogen as well as carbon.
Many substances are organic compounds. Polymers are organic compounds consisting of long chains of repeating patterns containing carbon and other atoms. Plastics, rubbers, and nylon are all organic polymers. Hydrocarbons are compounds containing only hydrogen and carbon, methane being a simple example. Crude oil is a sludge of various hydrocarbons mixed together. Propane, butane, and octane are well-known hydrocarbons used for fuel. Perhaps the most interesting type of organic compound is the biomolecule. Carbohydrates, proteins, lipids (fats), and nucleic acids (like DNA) are the most basic biomolecules. They, too, are polymers (except for lipids), being made of long chains of small, repeating chemicals that have bonded together. Biomolecules make up the chemicals found in the living cells that compose all living things.
Organic chemistry and biochemistry are very broad and thorough topics. They are far outside the scope of General Chemistry. Fortunately, your knowledge of General Chemistry is sufficient to begin the Wikibooks Organic Chemistry and Biochemistry if you are interested.
Silicon
[edit | edit source]
Silicon is found in semiconductors, the basis of all electronic devices. Its electron configuration allows it to donate or accept electrons. When pure silicon is "doped" with elements that have more or fewer electrons than silicon, the slightly-impure silicon becomes a semiconductor. These substances make up complex electronics by acting like a switch that can turn on or off depending on electrical signals.
Silicon does not exist uncombined in nature; it most commonly occurs in silica (including the very common rock quartz and most sand grains) and in silicates. Most silicates are insoluble. So-called 'magic rocks' react with dissolved sodium silicate in water reacts with the ions of some dissolved metal salts to form columns of rock-like silicates.
Silicon dioxide, a hard substance that melts only at high temperatures, is very different in its chemical properties from carbon dioxide, a gas until it freezes into dry ice. Molten silica can be cast as glass, a hard and useful material resistant to attack by almost all chemicals except fluorine, hydrofluoric acid, and strong alkalis. Glass is extremely useful in household containers and drinking utensils because it is resistant to chemicals, heat, and the attack of micro-organisms. Glass can be very clear if pure or containing certain chemicals, or it can take on attractive colors, making it a favorite material for art objects. Some small living creatures turn dissolved silica in the sea into their shells to create structure. A great variety of silicon compounds known as silicones have widespread and varied uses.
Silica is not strictly a poison, but as an abrasive it can damage tissues if eaten or breathed by grinding against them. Asbestos, a silicate (and derivative of silica) breaks down to silica especially in lungs and does great damage. |

Others
[edit | edit source]Germanium is another element used in semiconductors. Before transistors were in common use, germanium diodes were heavily used in radios.
Tin is considered a "poor metal". It has two allotropes at STP: grey tin and white tin. Grey tin has non-metallic characteristics, but white tin is metallic. Tin is used frequently as an alloy. "Tin cans" are actually steel cans with a tin plating to resist corrosion. Pewter is an alloy of copper and tin, containing mostly tin. Bronze is an alloy of copper and tin containing mostly copper. Solder is an alloy of tin and lead used for its low melting point for attaching wires.
Lead is a heavy, gray metal. It had a tremendous number of uses, but it is now known to be a neurotoxin if ingested. Water-carrying pipes were made out of lead, but they are now made from copper or plastic because lead could contaminate the water. Lead compounds especially if soluble in water (like lead acetate used as a preservative and sweetener of wines in Roman times) or stomach acids (like the lead oxide once used in paints) or in gaseous or liquid form (like tetraethyl lead once used in gasoline), and powdered lead are very dangerous. Use of lead in foodstuffs, paint, and vehicle fuels is now illegal almost everywhere.
Lead compounds and vapors are insidious poisons that accumulate in the brain and create learning disabilities and personality disorders. |
Lead-crystal glass has lead oxide in it but so tightly bound with silica that it can't escape easily. Pencil 'leads' are not lead at all, but instead harmless graphite (really carbon) bound with clay. Lead remains useful in automobile batteries (that contain necessary sulfuric acid much more dangerous than lead) and in nuclear use as a shield against radiation much more dangerous than lead metal.
| Lead oxides are easily reduced. | |
| Lead does not dissolve in hydrochloric acid or sulfuric acid, but it will dissolve in nitric acid because nitric acid is a strong oxidizer. | |
| Lead(II) oxide will form plumbite ions when added to basic solutions. | |
| Plumbites form lead(IV) dioxide when chlorinated. | |
| Adding lead(IV) dioxide to a basic solution will form plumbate ions. |