General Astronomy/Principles of Light
| General Astronomy | ||
| Motion and Gravity | Principles of Light | Telescopes |
Light is a medium of energy through which we perceive and interact with our environment. It is the visible frequency range of electromagnetic radiation which also includes invisible forms of electromagnetic radiation such as ultraviolet, infrared, and radio waves.
Like all electromagnetic radiation, light is transmitted by individual packets (or quanta) of energy known as photons. These photons are the units by which the combined forces of electricity and magnetism are communicated between other particles, such as the electrons associated with an atom. Depending on the circumstances under which it is observed, a photon can behave like a particle or as a wave. This principle is known as wave-particle duality.
Wave properties
[edit | edit source]The wave-like nature of electromagnetic radiation means it can be plotted on a graph as an oscillating electric and magnetic field at right angles to the direction of travel of the wave. The frequency of these oscillations is measured in the number of complete cycles per second, or hertz. The particular frequency of a photon places it somewhere on a spectrum of possible frequencies. This is referred to as the electromagnetic spectrum. The range of frequencies that form the visual spectrum lies between 3.8×1014 hertz (dark red) and 7.5×1014 hertz (violet).
The speed of light, given the symbol c, has been precisely measured as 299,792,458 m/s or approximately three hundred thousand kilometers per second and has been demonstrated to be constant in a vacuum. A vacuum being defined for the purposes of theory and experimentation as a volume of space that is essentially empty of matter. The speed of light is a fundamental constant of modern physics and remains constant regardless of the movement of the observer. Thus, for example, if you were somehow able to travel at half the speed of light, and you measured how quickly light was arriving from the front, you would measure it as arriving at the speed of light (3.00 x 108 m/s).
Since the speed of light is a constant in a vacuum, for a given frequency a photon will have a corresponding wavelength, or the distance between the crests of the wave. The frequency and wavelength of light are directly related by the following equation:
where c is the conventional symbol for the speed of light, usually in meters per second, f is the frequency of light in hertz, and λ is the wavelength in meters.
Given the speed of light as 3.00 × 108 meters per second, then the wavelength range for the visual spectrum is about 400 to 800 nm, or nanometers. (A nanometer is 10−9 meters, or one-billionth of a meter.)
The shorter wavelength of 400 nm corresponds to the greater frequency, and is located at the blue end of the visual spectrum. Likewise the longer wavelength of 800 nm belongs to the red end of the spectrum. The actual energy of the photon increases with decreasing wavelength (or increasing frequency.)
Einstein won the Nobel Prize for applying Planck's theories to electromagnetism.
Intensity
[edit | edit source]The intensity of a source of radiation is the energy it emits per unit of surface area per unit of time and has the units of Joules/(meter2 x second). As the energy radiated by a spherical surface, I0, moves away from that surface the radiation intensity decreases as the inverse of the distance squared (I=I0/d2) because the radiation spreads out. In other words, the perceived intensity of a light source by an observer is inversely proportional to the distance from the light source squared. Thus for each doubling of distance from the source, the intensity drops by a factor of four, or 2 × 2.
The brightness of stellar objects, such as a star, are determined by the amount of light they radiate and their distance from the Earth. A bright star in the sky might actually be much more distant than an a dimmer star, but because it is more intense and radiates a greater amount of light it appears to be closer.
Astronomers record the light intensity of a stellar object as a numerical magnitude. The magnitude is a number on a logarithm scale that has been standardized, so that 5 steps in magnitude is equal to a multiple of 100 in intensity. In addition, the value of the magnitude increases as the intensity of the light source decreases.
Thus a star of magnitude 2.0 is dimmer than a star of magnitude 1.0. A magnitude 1.0 star is also 100 times as bright as a magnitude 6.0 star. Each +1.0 magnitude increase is the same as dividing the intensity by 2.512.
The reference point for the magnitude scale is set to zero. At one time this was based on the star Vega, or α Lyrae. The brightest star Sirius (α Canis Majoris) has a magnitude of −1.46. The limiting magnitude for a typical person's unaided eye is considered to be 6.0. However people have observed stars fainter than this under good conditions. Much fainter stars can be seen by using the larger collection area of a telescope and the extended recording ability of a camera.
Reflection
[edit | edit source]A mirror is a flat or curved surface usually made out of a highly-conductive material, such as a metal. When light interacts with a mirrored surface, it undergoes specular reflection. That is, a beam of light striking the mirror is reflected in only one direction. This direction is determined by the law of reflection, which states that the angle with the surface at which the light is reflected is the same as the angle with the surface at which it approaches.

|
| In this illustration, the reflected light rays reaching the eye from an object produce the illusionary appearance of a reversed-image object behind the mirror. |
The movement of a photon with respect to the mirror consists of two components. The first is the proportion of the movement parallel to the mirror, and the second is the portion perpendicular to the mirror. After the reflection, the portion parallel to the mirror is unchanged. However the portion perpendicular is now in the opposite direction. That is, it effectively "bounces" off the surface almost as a rubber ball bounces off the ground.
When light interacts with a surface that is not reflective, a portion of the light is absorbed by the surface and the remainder is scattered in random directions. This type of reflection is called diffuse, and it is responsible for the illumination effect of ambient light.
The portion of light absorbed by a surface is termed its albedo. The lower the albedo rating, the less light it reflects in a diffuse manner. A surface with a low albedo rating appears dark to an observer, while a high albedo rating appears light. The albedo rating of a surface can tell an astronomer something about the nature of the surface. For example, a surface covered with carbon soot will have a low albedo, while an icy surface has a higher albedo.
Refraction
[edit | edit source]When light passes at an angle through a transparent medium, the material causes the photons to change direction slightly. This change in angle is called refraction, and the angle by which the light is bent is determined by the index of refraction of the material.
The index of refraction of the two materials that the light passes between can be used to determine the change in angle by means of Snell's law. For materials with indices of refraction n1 and n2, the angle in the first material θ1 determines the angle in the new material θ2 as follows:
Here are the indices of refraction (at a wavelength of 589 nm) for some common transparent materials relative to a vacuum:
Material Index Air 1.003 Water ice 1.331 Water 1.333 Quartz 1.46 Crown glass 1.52 Dense flint glass 1.66 Diamond 2.419
where the index of refraction for air is at sea level with a temperature at the freezing point of water, and the water is at 20 °C.
Spectrum
[edit | edit source]For a given transparent material, such as glass, the refraction of light varies with frequency. A white light consists of photons of various energies. The red photons in the light will be deflected at a different angle than the blue photons.
If the light passes through a transparent material with parallel sides, such as a sheet of glass, the beam will emerge at the same angle as it entered. However when the two sides are not parallel, the angle will vary depending on the frequency. This is the principle behind the prism. A glass prism is used to separate the photons from a light source into a spectrum of frequencies from red to blue. A similar principle is what creates a rainbow as the light from the sun passes through droplets of water.

|
| The index of refraction varies by frequency, causing parallel, monochromatic light rays from the left to emerge from the prism at different angles. |
An instrument specifically designed to display the spectrum of a radiating object, such as a star, is called a spectroscope.
The early spectroscopes were constructed using a series of prisms that would successively spread the spectrum further apart. The problem with this arrangement, however, is that each of the prisms would absorb some of the light passing through. This limited the brightness of the objects that could be observed. An instrument called a diffraction grating, which was a mirror with a series of ruled parallel grooves, used the principle of diffraction to produce a spectrum with only minor loss of intensity.
Isaac Newton discovered that a light beam can be diffracted only so far, and no farther. The diffraction can be recombined into white light.
Lens
[edit | edit source]The lens takes advantage of the property of refraction to bend the light from a distant object and to make it appear closer (or more distant). A lens is, in a simplified sense, a prism that has been "wrapped" around in a circle, so that the light is bent symmetrically.
Because light of different frequencies is bent at different angles, however, the point at which the light comes to a focus varies with frequency. An observer looking through a lens would see light sources near the edge have a rainbow-like appearance. This is called chromatic aberration.
To adjust for this variation in the focus by frequency, opticians typically use combinations of lenses made of different materials (with differing indices of refraction). Judicious use of materials and lens shapes will result in a lens that focuses all the light at the same distance, producing a good quality image that does not suffer from chromatic aberration.
Magnification
[edit | edit source]When you observe an object nearby, it subtends an certain angle within your sight. That is, if you had an imaginary line running from the top of the object and your eye and a similar line from the bottom of the object to your eye, there would be a certain angle between these lines.
As the object recedes into the distance, the angle it subtends across your sight steadily decreases until it becomes nearly a point. The imaginary lines from the top and bottom of the object are now nearly parallel. In fact, for an astronomical object such as a star, these lines are essentially parallel.
In order to enlarge the appearance of an object, it is necessary to modify the paths of the incoming light rays so that they are no longer parallel but instead arrive at an angle as they enter your eyes. The eye then perceives the object as if it were much closer.
There are two common means for causing the parallel light rays to converge in this manner. The first involves the use of a curved, concave mirror. The second takes advantage of the refraction ability of materials such as glass to redirect the light inward at an angle.
The shape of glass needed to accomplish this is a convex lens. The portions of the lens near the center need little curvature since they will are required to bend the light only slightly toward your eye. At the edges of the lens, however, the light needs to be bent at a sharper angle, so the sides of the lens become bent toward each other like a prism. Overall the sides of the lens form a smooth curve that gradually increases in slope toward its edges.
A well-made convex lens will cause the parallel light from a distant light source to focus at a point. When there are multiple such light sources, they are each focused at a point on a plane, known as the focal plane. The human eye can perceive the image of this plane, and the result is a magnification of the view. If the images do not focus on a plane, then the image will appear blurry.
Diffraction
[edit | edit source]Another wave-like property of light is a tendency to bend and spread whenever it meets an obstacle. Any beam of light will also tend to spread with distance, so that it becomes impossible to maintain a tight beam of an arbitrary length. The property of diffraction is what limits the resolution of a distant object.
When a beam of coherent light, such as that produced by a laser, is passed through two slit openings, the light radiates from the slits like ripples in a pond. The semi-circular ripples from the two slits interact with each other, sometimes adding together their wave heights and at other times cancelling each other out. This is called constructive and destructive interference. If a screen is placed in the area where these ripples interact, alternating bands of light and darkness would appear.
Resolution
[edit | edit source]The resolution of a viewing instrument is a measurement of how well it can be used to distinguish two points that are very close together. For example, the two points could be the two stars in a binary star system. In astronomy, resolution is usually measured in seconds of arc. The resolution can vary depending on a number of environmental and quality conditions, but it is always limited by the aperture of the observing instrument. That is, there is a best possible resolution that any particular telescope can achieve. To get better resolution, a larger aperture is needed.
To see why this is so, imagine a telescope that consists of only two vertical slits separated by some distance, with a viewing screen behind. When the light from a distance star enters this telescope, it passes through the slits and forms an interference pattern on the screen. The distance between the light and dark bands is proportional to the wavelength of the light and inversely proportional to the distance between the slits. Thus increasing the separation of the slits will reduce the width of each band.
Now suppose there are two stars. They will both form bands of light and dark light on the screen, which may overlap. The closer the two stars are two each other, the closer their interference bands approach until they become indistinguishable. But if the separation of the slits is increased, then the bands become narrower and the stars can be distinguished again. This is the principle behind the interferometer.
Interferometer
[edit | edit source]In an ordinary telescope, the resolution is determined by the aperture. In this respect, a telescope can be thought of as a whole series of slits allowing light through, with the light at the outer edge providing the maximum resolution. The resolution of the telescope can be improved by adding a set of mirrors outside the maximum aperture that collect peripheral light rays, and effectively increase the aperture.
Similarly, two or more telescopes can be configured to work together and provide an aperture at least equal to the separation of their collecting surfaces. This setup is called an interferometer, because the images from both telescopes are integrated through a process of diffraction interference. Radio telescopes have successfully used this technique for many years to achieve very high levels of resolution. Optical interferometers are more difficult to build due to the requirements for extreme precision and the need to dampen out any vibrations.
Reflection gratings
[edit | edit source]Reflection gratings are surfaces that have been very precisely ruled with a series of parallel grooves. The grooves have a saw-tooth pattern, with each groove consisting of a long flat surface machined at a slight angle, with a sharp step at the edge. Each of the grooves is very narrow, with about 600 lines per mm (15,000 per inch).
As light is reflected from each of the grooves, it is slightly behind the light from the adjacent grooves. This difference produces an interference effect that reinforces the light at certain angles and cancels out the light at others. The grating is very efficient at destructively interfering with the light except at one particular angle, where the light constructively interferes and produces a peak intensity. The angle of this peak varies by the wavelength of the light, so a spectrum is produced.
Polarization
[edit | edit source]In addition to a direction of travel, a photon is composed of an electric and magnetic field. These lie at right angles to each other and to the direction of travel. This is known as a transverse wave. These perpendicular fields give the photon an orientation. The fields of each photon will maintain their orientation while traveling in a vacuum. Fields of this type are called plane-polarized.
Normally light from a source consists of a large number of photons that have a random polarization. However, it is possible for a number of the photons to become oriented in the same direction, becoming polarized. This coherent orientation can be detected by means of a sheet of polarizing material. When the sheet is oriented in the direction of the polarization, the polarized light passes through. As the sheet is rotated, it transmits a decreasing portion of the polarized light until finally, at right angles to the plane of polarization, it blocks all of the polarized light.
Light can become partly polarized by reflecting from a surface, such as sunlight reflecting off a pool of water. Reflected sunlight provides a source of glare for somebody driving a vehicle. Because this light is partially polarized, the use of polarized sunglasses helps reduce glare by blocking the polarized light preferentially.
Astronomers can examine a stellar light source to determine whether it is a source of polarized light. The presence of polarization is an indication of certain physical properties in effect at the source of the light, or along the line of sight of the light rays. For example, a magnetic field can polarize a light source, as can the acceleration of an electron to a velocity near the speed of light.
Spectral lines
[edit | edit source]When an atom absorbs a photon of light, the energy is forces the absorbing electron in the atom into an excited state. The electron changes its behavior, effectively becoming more energized and entering a new orbital pattern about the nucleus. A sufficiently energetic photon, or a combination of photons with enough energy, can even knock the electron from the atom. The atom then becomes ionized and gains a net positive charge.
Due to the quantum nature of small particles, the changes in energy allowed for an electron in an atom is fixed to very specific amounts. When a photon of just this energy is captured by the electron, it is must jump to a new and higher energy level. Thus each atom has a specific set of energy bands where it will favorably absorb photons, depending on the current energy states of its electrons.
When a white light is passed through a gas composed of the same type of atom, those atoms will tend to absorb light at those frequencies that match the energies needed for their electrons to jump to a new level. An observer on the other side of the gas who looks at the spectrum will see dark lines where these energies have been absorbed. Likewise an observer looking at the gas from another angle will see bands of light where those same energy frequencies were emitted by the atoms.
This property of selective absorption of light at specific bands is important in astronomy because it allows an astronomer to determine the chemical properties of a distant object. A star, for example, will radiate a spectrum with strong or weak absorption bands, which are determined by the quantities of different gases on its surface. The science of recording and measuring these lines is called spectroscopy.
Doppler shift
[edit | edit source]As an object moves toward us in space, it may radiate light in our direction. The velocity of the light we receive does not change. However, during the time interval between each of the peaks in the light wave it is transmitting, the object has moved slightly closer toward us. Thus the wavelength grows shorter and appears more blue than normal. Correspondingly, an object moving away from us will have its wavelength stretched out, making it appear more red.
This red-shift or blue-shift property has a number of important applications in astronomy. It can be used to measure the velocity with which a distant object, such as a galaxy, is moving toward or away from us. For objects that are rotating, we can measure the rate of rotation by comparing the blue shift on the edge rotating toward us to the red shift of the edge moving away. We have also discovered binary stars by the regular oscillation of the spectrum toward the blue or the red as the star orbits its companion.
Spectrometry and Photometry
[edit | edit source]Spectrometry involves looking at the spectra of light. Spectra are what you get when you take light from a source and spread out the colors by passing the light through a prism or over a grating, and then looking at the amount of light at a certain wavelength. There is a huge amount of information that you can get from doing this.
So let's take a spectroscope and point it at something like a fluorescent light bulb or a nebula. The thing that you will see is that rather than a continuous rainbow of all colors or wavelengths, the light is actually a combination of light from different well defined wavelengths. You will see lines.
The reason for these lines is that the electrons in the gas in the fluorescent lights can only be at certain energy levels. When you do something to energize the gas in a fluorescent bulb, the electrons in the atom's gas move to higher energy orbits, which are known as excited states. They stay in those excited states for a length of time ranging from milliseconds to seconds. When the electrons drop from the high energy states to lower energy states, then they will emit light at a wavelength that has an energy (and a corresponding wavelength) equal to the difference between the two energy states. This is known as an emission spectrum.
The detected spectra gives insight into the composition of the object emitting the spectra. Every element and material has its unique set of energy levels and unique lines, and by comparing those the lines of emission spectra with those of known elements, it's possible to discover the object's composition.
When you add energy to an atom, the electrons move to a higher energy state. As the electron relaxes and moves down the energy states, it emits a particle of light for each transition that it makes. Since every energy has a particular color associated with it, each transition puts out light at a single wavelength. The time between stimulation and re-emission is very rapid (in like a microsecond), but there are some materials in which the transition from a high energy state to a low energy state takes a long time. An example of this is glow in the dark stickers. When you expose it to light, it kicks some of the electrons into high energy levels, and it takes seconds to minutes for the electrons to revert to their original state.
One can discover other things about an object from its spectrum. For example, as you increase the temperature, you end up with more and more electrons in higher energy states and this affects the spectrum in that you end up with stronger lines. But if you increase the temperature past a certain point, the electrons leaves the atom completely and the lines become weaker.
You can also discover the pressure and density of the object. As the pressure and density increase, there is a increased chance that particle interactions will change the electrons' energy states to higher or lower level. This causes the lines to grow wider since there is a higher chance that the electron won't start and finish at a particular energy level.
If you increase the pressure and density enough the electrons no longer have enough time to stay at a certain energy level, and so the lines broaden to form what is known as a continuous spectrum, A continuous spectrum is emitted by a solid, liquid, or high pressure gas. Because the electron is no longer restricted to certain energy levels and certain wavelengths, the electron will often emit a low energy infrared photon rather than a photon of light. As a result something that is emitting a continuous spectrum (like a light bulb, specifically an incandescent light bulb) will emit much of its energy at lower frequencies (called heat) compared to something that is emitting a more discrete spectrum (like a fluorescent light bulb). Since energy is conserved, a fluorescent light bulb emits almost all its energy at a few wavelengths very efficiently while an incandescent light bulb emits much of its energy as heat. Hence, a florescent bulb will convert electrical energy into light more efficiently.
There is one more type of spectrum which is very common. If you expose a gas to light of different wavelengths and one of those wavelengths happens to match a difference in energy levels in the gas, it will absorb the light at that particular wavelength. So, if you have a source of a continuous spectrum pass it into cool gas in front of it, you produce what is known as an absorption spectrum. Most stars emit absorption spectrum as the cool upper layers of the stars absorb lines from the light emitted by the hot lower levels of the star.
So far we have been talking just about visible light, but the principles of spectroscopy apply to other types of electromagnetic radiation, of which visible light is just a small slice in the overall range of wavelengths. You can have gamma ray or X ray spectra (at shorter wavelengths than visible light) as well as microwave and infrared spectra (at longer wavelengths than visible light). The big difference has to do with what generates the radiation. The energy differences between different states of an atom typically are the energy of a particle of visible light. An X-ray photon will knock the electron right out of an atom, as a result an X-ray can't be generated by electrons transitioning between atomic energy levels. However, X-rays are generated when atomic nuclei transition between different nuclear energy levels. Conversely, microwave radiation can be generated when molecules move between energy states as they "wiggle." So by observing microwaves you can detect cool clouds of molecular gas by detecting microwaves in the spectrum. Conversely by sending microwaves into something that contains water the molecules will be induced to "wiggle" or in other words heat up. At the same time, the microwaves will pass through things (air or ceramic) whose energy levels don't match the microwaves. So if you put something like a cup of coffee in a microwave oven, all of the energy will be absorbed by the coffee and not by the cup or the air.
One final thing about spectroscopy. Spectrographs are affected by so many things and that every object out there has a different spectrum, and understanding what affects spectrographs and how to glean this information from spectrographs is an important part of astronomy.
Discussion questions
[edit | edit source]- The stellar classification system from hottest to coolest is OBAFGKM with A being the star with the strongest hydrogen line, B being next strongest and so forth. Why is the stellar classification in this order rather than the more logical order of temperature? Discuss a case from your experience in which a similar reason has led to seemingly odd classification systems.
- Identify three objects and tell me whether they would result in an emission, continuous, or absorption spectrum. Also tell me what you would see if you pointed a spectroscope at you. Would you see an emission, continuous, or absorption spectrum?
- What type of spectrum do you suppose an LCD puts out? What about gold? What about a microwave oven? What about you?
- Using your knowledge of advances in photography, how do you suppose an astronomer takes a spectrum differently today than in 1920? What about 1850? How do you think the Internet can be used to help astronomers take spectra?
- Why do you think it is so tough to create a good looking fluorescent light bulb and how do you think that they do it.
- If I stand in front of 100 watts of radio waves or light waves, nothing bad happens to me. But if I stand in front of 100 watts of gamma rays or X-rays, bad things will happen to me. Why?
Violet scatters the most, and red the least.
Definition
[edit | edit source]Spectrum is a word that has taken on a broad meaning in English, first used by scientists such as Isaac Newton in the 1600s to specify the range of colors obtained by passing sunlight through a glass prism, or produce through the natural mechanism of a rainbow. Today it is applied in almost any situation for a broad range of values. Specifically in physics and astronomy it still denotes the range of colors of visible light, but also includes invisible forms of electromagnetic energy ranging from very long wavelength radio waves to the ultra short wavelength gamma radiation.
Producing a Spectrum
[edit | edit source]Color is analogous to wavelength when we speak of visible light. There are relatively long wavelengths of red light and relatively short wavelengths of blue and violet light. These wavelengths are also indicative of temperature for a heated body; red is cooler while blue is hotter. White light, such as that from the Sun, is not composed of a single color or wavelength, but of a mixture of many colors or wavelengths, which the eye interprets as white.
While all wavelengths of light travel at the same speed through a vacuum, the speed of different wavelengths varies as light passes through a transparent medium such as glass, water or even air. As light passes from one medium (such as air) into another medium (such as glass), its speed changes according to the index of refraction of the two media. In the case of this example, light slows down as it passes into the glass. Blue or violet light is slowed slightly more than red light as it passes from a medium of lower refractive index to one of higher refractive index. This, along with the particular shape of a glass prism, acts to bend or disperse light, spreading the colors out. Since blue light is bent more than red light, the original mixture of light is spread out into its constituent colors to form a spectrum, somewhat like an artificial rainbow.
“The brightness of a star is also dependent on its temperature, and the temperature will have an effect on the spectrum the star emits. If two stars with identical spectra are observed, and the distance of one of the stars through parallax measurement is known, their brightness can be compared. The variance in brightness is attributable to the difference in distance. Using the inverse square law, the distance of the star whose distance was previously unknown can then be determined. Stars can give off radiation not only in the visible spectrum but also as radio waves, x-rays, and gamma rays. All of these different parts of the electromagnetic spectrum can be used in conjunction with the techniques already discussed to make astronomical measurements.”
Types
[edit | edit source]Spectra types are
- continuous, which are broad bands created by a incandescent solid (such as the red hot element of an electric stove), liquid (such as molten lava) or a high-pressure gas (such as the surface of a star).
- emission, characterized by narrow bright lines, created by an excited, low-pressure gas. Examples of emission spectra sources are a comet's coma and tail, and the Rosette nebula.
- absorption, characterized by narrow dark lines, created by a continuous spectrum that is passed through a low-pressure gas. This is seen in the spectra of the Sun and stars, and is caused by light absorption in the cooler, lower pressure gas atmosphere of the star.
By studying spectra, astronomers can discover many things about stars, most specifically the chemical elements found in the star. “The record of wavelengths (or frequencies) of electromagnetic radiation absorbed by a substance; the absorption spectrum of each pure substance is unique.”
Most stars exhibit absorption line spectra, but a few rare stars show emission lines. Wolf-Rayet stars have emission spectra caused by UV (ultraviolet) radiation from a hot star passed through low-pressure gas. Certain nebulosity or gas clouds also exhibit emission lines. Spectral lines also are detectable in non-visible light such as ultraviolet and microwaves.
Newtonian Physics
[edit | edit source]Isaac Newton formulated the Universal Law of Gravitation, the Laws of Motion, and calculus. The Universal Law of Gravitation is summed up in the formula
where and are two masses, in kilograms, and is the gravitational constant . is the distance between the centers of the two masses, in meters. is measured in Newtons.
Work is calculated with the formula
where is work (measured in Joules), is force (measured in Newtons), and is distance (in meters).
Kinetic energy (energy of motion) is calculated by the formula
where is mass (in grams), and is velocity.
Newtonian relativity: A man is walking at 1 km/h, and he throws a ball at 3 km/h. To get the speed of the ball, simply add speeds: .
The First Law of Thermodynamics states that energy can be neither created nor destroyed, only transformed into one of its two forms: energy and matter.
The Four Forces
[edit | edit source]There are four forces in the universe: gravity, which holds together galaxies and other massive structures; the electromagnetic force, which holds atoms together; the nuclear force, which holds atomic nuclei together; and the weak force, which is concerned with the transmutation of elements and radioactive decay. The nuclear force is the strongest, and gravity is the weakest. Without these forces, the universe would disintegrate.
The gravitational force causes mass to attract mass. More massive objects have a stronger gravitational field.
The electromagnetic force can be summed up by the phrase "opposites attract" and allows atoms to bond with each other creating the great variety of compounds that make our experience possible. The nucleus, with its positive charge, attracts negatively charged electrons. The electro- static force is calculated according to Coulomb's Law.
The power of the nuclear force depends on distance. At a distance of between one and 2*10−15 meters, the force is attractive. However, if distance is too close (less than 10−15 meters), the force is repulsive; and at distances greater than 2*10−15 meters, the force diminishes to zero.
Atoms
[edit | edit source]The atom was first postulated by the Greek philosopher Democritus. He believed that matter could not be split indefinitely. He believed that all matter was composed of connected particles which could be split apart, but could not be split themselves. These indestructible pieces are called atoms. The word comes from the Greek atomos, which means "uncuttable"---a (not) + tomos (to cut). [1]
The Periodic Table of Elements was created by Dmitri Mendeleev in 1869 (revised in 1871). The manmade elements are radioactive and have short half-lives.
Nuclear reactions are categorized as critical or supercritical. A critical reaction is one neutron in, one neutron out. A supercritical reaction is one neutron in, three neutrons out---releasing a terrific amount of energy. Supercritical reactions are used in atomic weaponry.
Nuclear fusion is a great source of energy, but requires temperatures of 1,000,000 Kelvins.
Particles
[edit | edit source]All atoms are composed of particles. Particles are characterized by mass, charge, and spin. A particle's spin is right-handed (counter-clockwise) or left-handed (clockwise).
At the center of the atom is the nucleus, which contains a number of protons and neutrons about which the Electrons orbit. The force that keeps the electrons in orbit is the electric force; the force that keeps the nucleus together is the nuclear force.
In a neutral atom, there are equal amounts of protons and electrons. For example, Helium has two protons in its nucleus and two electrons orbiting its nucleus. It also has two neutrons in its nucleus. When there is more electrons than protons, or vice versa, then the atom is called an ion and it is more reactive with other ions and atoms because it has an overall net charge associated with it.
An electron has a negative (-) charge, a proton has a positive (+) charge. Neutrons, neutrinos, and photons have no charge. The most massive of these is the neutron; it can decay into a proton, electron, and a neutrino. Particles are made up of quarks. The six types of quarks are up, down, strange, charmed, top, and bottom.
Antimatter was predicted by Paul Dirac. Every particle has an anti-particle, with the same mass, but an opposite charge and spin. There are anti-electrons/positron, anti-protons, anti-neutrinos, and anti-photons. (The anti-photon has the same spin as the photon.) When a particle meets its anti-particle, the result is mutual annihilation, and the creation of energy. The opposite is also true: when two photons meet, matter is created. This creation of matter is called "pair production".
If there are antimatter stars; their light would be identical to that of matter stars, because the anti-photon is the same as the photon.
Astronomy studies the flow of energy, and forces. Energy comes primarily from two sources: gravity from gaseous clouds collapsing to form stars and planets; and nuclear energy. The fusion that makes stars burn is one type of nuclear energy; another is the radioactive decay that heats the cores of planets.
Earth has a magnetic field. The core has a current. This field causes the Aurora Borealis.
The relationship of frequency and wavelength to the speed of light is shown in the formula
where is frequency, is wavelength, and is the speed of light.
A photon is a discrete packet of light energy. To calculate the energy of a photon, use the formula
is Planck's constant:
is frequency, which is in units of
Einstein's famous equation, , demonstrates that mass and energy can be converted into each other. is energy, is mass, and is the speed of light, .
Spectroscopy
[edit | edit source]"The one thing that man will never know is the chemical composition of the stars." ---Auguste Comte, 19th century philosopher
He was wrong!
Kirchoff & Bunsen discovered that individual elements burn with different colors. Different colors correspond to different wavelengths of light. The colors given off can be recorded on a photographic plate. This is called the elements' emission spectrum and it is unique to each and every known element. Therefore, any known element in the laboratory can be determined by investigation of its "spectra".
An explanation of why different elements give off different wavelengths of light is needed to explain how the composition of stars can be determined. An individual element has a unique number of protons. If you were to follow the periodic table from left to right, you would find that for the first few lines the atomic number increases by one each time. Hydrogen is the smallest element as it has one proton. Helium is the next smallest, as it has two protons, and so on.
Each of these elements therefore has a different amount of electrons and protons. Assuming these elements are all neutral, each successive element consists of one more electron than the previous element. i.e. helium has two electrons, and hydrogen has one.
Electrons orbit the nucleus of an atom. They can be described as having an energy level associated with them. The electrons in a particular element can only occupy a specific energy level or shell. When elements are heated, there is an input of energy, which is distributed to these electrons and they therefore move to a higher energy level. When this electron falls back to its original energy level, the energy gained by heat must be lost. The electron loses this energy by emitting a photon(a packet of light).
This photon will have exactly the right amount of energy needed to enable the electron to fall to its exact original state. This energy can be calculated using E=hf, where E is the energy, h is Planck's constant, and f is the frequency of the individual photon. While it seems odd that a particle would have a frequency, it does due to wave-particle duality.
From above, it can be seen that each element, because each has electrons that only occupy certain energy levels, that the frequency of the photon emitted can only have certain values.
From the equation c = f * lambda, where c is the speed of light, which is nearly always the same, f is the frequency and lambda is the wavelength, it can be seen that because c is constant, each element, by only emitting photons with certain frequencies, gives off photons with certain wavelengths and therefore colors.
It is impossible to use the laboratory technique of defining elements by using their emission spectrum because the light that we receive from the stars is a combination of colors. There is however, another way. If we were to view the emission spectra of the sun, for example, there would be no signature "barcodes" of individual elements, rather, there would appear a continuous spectrum, like a rainbow on paper. This "continuous" spectrum will have a few black lines, where the wavelength of light has been absorbed rather than been emitted by the Sun's photosphere. It is from these, that we can deduce a stars chemical composition.
It was discovered that the black lines in the continuous spectrum of a star corresponded exactly to the emission lines of certain elements. Their presence in the star is suggested because those elements emit light of the same wavelength that is absorbed and hence, shows as a telltale black line. It receives this light in a concentrated beam but emits it in all directions. If you imagine the light rays as a 20 pack of javelins hitting an element in a certain place, the element will throw these javelins back out individually, into the surroundings so that the amount of light emitted in the direction of Earth is minute or non-existent, and hence we observe dark lines in the emission spectrum.
The Quantum Model of the Atom
[edit | edit source]Quantum physics is a relatively new branch of physics that deals with very small objects, such as atoms and quarks. It follows different rules than classical (or "Newtonian") physics. While Newtonian physics assumes that energy can be continually divided and holds that an object can have an arbitrarily small amount of energy, quantum physics deals with objects that emit or absorb discrete packets of energy known as quanta that cannot be further divided. Classical physics assumes a continuum whereas quantum physics assumes the universe is discrete.
Max Planck is considered the "father of Quantum Theory".
In 1913, Danish physicist Niels Bohr used Ernest Rutherford's research on the atomic nucleus and Max Planck's quantum hypothesis to create a quantum theory of atoms. This theory stated that an atom's electrons move only in definite orbits. When a hydrogen atom emits an Hα photon, the electron drops to a lower orbit. When a hydrogen atom receives a photon, it jumps to a higher orbit.
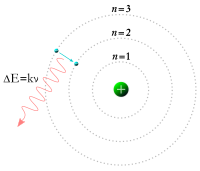
The hydrogen spectrum has been studied for ultraviolet (the Lyman series) and visible light (Balmer series). In the Lyman emission series, the electron drops from a higher orbital to the n=1 orbit. In the Balmer emission series, it drops from a higher orbital to the n=2 orbit. (n=1 is the lowest energy state, or orbit, of an electron, called the principal quantum number.) The energy change produced as an electron moves from n=2 to n=1, results in the emission by the electron of a photon of energy 10.2 eV and appears in the ultra-violet part of the spectrum. The energy change produced as an electron moves from n=3 to n=2, results in the emission (H-alpha) by the electron of a photon of energy 1.89 eV and appears in the red part of the spectrum.
The energy levels in a hydrogen atom, can be calculated by:
where is the electron's orbit.
In 1929, Prince Louis de Broglie won the Nobel Prize for his theory of matter waves.
Albert Einstein and the Theory of Relativity
[edit | edit source]Einstein's Principle of Equivalence demonstrated that gravity causes space to curve. He discovered that the curvature of space determines how matter will move. Thus gravity can be thought of as a consequence of the "shape" of the universe rather than a force vector. This is Einstein's Law of Motion. Under the theory of General relativity light should also be affected by gravity. This phenomenon has been observed by studies of gravitational lensing. Most of the information about stars is obtained from studying electromagnetic radiation. Interstellar dust can be observed to get information, as well.
Electromagnetic radiation includes UV (ultraviolet), radio waves, and X-rays. There are two kinds of waves: longitudinal (such as the way sound travels), and transverse (the way light and other electromagnetic radiation travels). Transverse waves are measured by their wavelength and frequency. Wavelength is represented by the Greek letter (lambda). Longer wavelengths make shorter frequency.
The formula for frequency is
where is the speed of EM radiation, meters per second.
Astronomers can learn a lot about distant objects by analyzing spectra. The 3 types of spectra are continuous, emission, and absorption. A continuous spectrum comes from a high pressure blackbody, or thermal source (such as a light bulb). An emission spectrum has bright lines. It is caused by cool, low-pressure gas. An absorption spectrum has dark lines it. It appears when a blackbody's light passes through a cool, low-pressure gas. For example, our sun's surface emits a continuous spectrum, but it becomes an absorption spectrum once it reaches us, because it has passed through the sun's atmosphere. Because of this, all normal stars have absorption spectra.
Since each element has a unique spectrum, astronomers can determine the chemical composition of stars by analyzing their spectra. They can also determine the pressure of the object by the type of spectrum: continuous (high pressure), or emission (low pressure). Astronomical spectroscopy is one of the most powerful tools used by astronomers to gain a fundamental understanding of our universe. Astronomical spectroscopy is a technique in which the absorption and emission of electromagnetic radiation from stars and other celestial objects are studied. In order to be able to interpret and predict absorption and emission spectra from celestial bodies, one must have a fundamental understanding of molecular emission and absorption. Molecular emission and absorption is the process in which photons are emitted and absorbed when a molecule changes quantum energy states. By studying molecular emission and absorption, the chemical composition, physical properties, and velocities of astronomical objects may be measured.
Quantum Mechanics
[edit | edit source]One of the key concepts from quantum mechanics essential to the understanding of molecular absorption and emission is the fact that molecular energy is quantized. In other words, molecules can exist only in specific quantum states with each quantum state having a set amount of energy. The quantized energy stored in a molecule can be thought of as the sum of energy stored in three distinct modes: (1) rotation, (2) vibration, and (3) electronic:
Since the internal energy levels of the molecules are quantized, discrete differences in energy are observed when molecules change quantum states. These transitions correspond directly with the energy of emitted or absorbed photons in discrete spectra. As stated earlier, emission is the process by which a molecule changes quantum states from a higher to a lower quantum state by the release of a photon. Absorption, on the other hand, is the process in which a molecule changes quantum states from a lower energy level to a higher energy level by absorbing a photon.
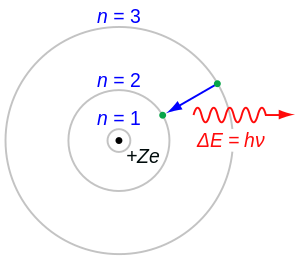
The total change in energy associated with a molecular transition (emission or absorption), can be described by the following:
In this equation, is the energy of the photon which is equal to the difference in energy associated with the molecular transition between two quantum states, is the frequency of the corresponding electromagnetic wave, and h is Planck's constant. This relation, known as Planck's Law, is important because it links the concepts of thinking of radiation as both a particle and a wave. Figure 1. illustrates the concept of emission due to the electronic transition of electrons orbiting the nucleus of an atom. It should be noted that even though this picture shows electronic transitions for an atom, the same processes govern electronic transitions in molecules. Using Planck's Law, the total change of internal energy in a molecule can be described as:
This equation states that the total internal energy of a molecule is the sum of the changes in rotational, vibrational, and electronic energy. From quantum mechanics, it can be shown that the quantum energy levels for the different modes of internal molecular energy have different spacing. Electronic states are more widely spaced than vibrational states, which are more widely spaced than rotational states. Because of this different spacing, changes in the different modes of internal energy lead to absorption and emission of electromagnetic energy at different wavelengths. Changes in rotational energy lead to microwave transitions, changes in rotational and vibrational energy (vibrotational) lead to infrared transitions, and changes in rotational, vibrational, and electronic energy (rovibronic) result in ultraviolet transitions. It should be noted that changes in vibrational energy are often accompanied with changes in rotational energy and changes in electronic energy are often accompanied by changes in vibrational and rotational energy.
Emission and Absorption Spectra
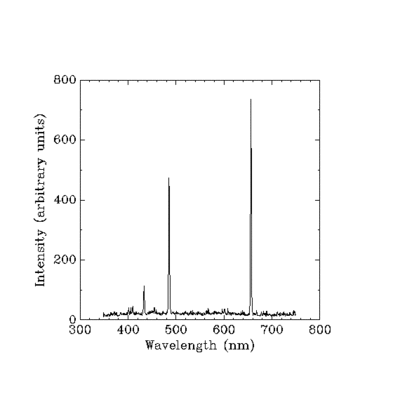
[edit | edit source]The absorption and or emission spectra of a molecule generally consist of a number of "lines". These lines correspond to the discrete differences in the internal energy modes of a molecule. In other words, a line is the part of a spectrum that corresponds to a transition from one quantum state to another. Groups of lines can in turn comprise a vibrational band. The positions, strengths, and shapes of lines can give accurate physical characteristics about the molecules undergoing internal energy transitions. The position of spectral lines reveals various molecular parameters such as internuclear spacing and molecular bond angles. Line strengths and shapes, on the other hand, can reveal the composition, temperature, pressure, and velocity of molecules in a radiating gas. This concept is illustrated in figure 2 which shows the emission spectra of hydrogen plasma.
The governing law for absorption spectroscopy that links various molecular properties with the amount of light absorbed in a gas medium is known as Beer's Law.
In this equation, is the fraction of light transmitted through a gas medium, is the frequency of the EM radiation being transmitted, is the spectral absorption coefficient, and L is the absorption path length. A diagram of Beer-Lambert absorption is presented in Figure 3. The spectral absorption coefficient is given by:
where is the "strength" of the transition, is the "lineshape function", and is the partial pressure of the absorbing gas species.
Diatomic Molecular Spectra
[edit | edit source]The interaction electromagnetic radiation has with matter can be explained through three main types of interactions: electronic dipole moment, induced polarization, and elastic scattering. Interactions with the electric dipole moment result in changes in the absorption and emission of radiation while induced polarization and elastic scattering are a direct result of how a molecule scatters photons. Many diatomic heteronuclear molecules have a permanent dipole. A dipole has a positive charge on one end and a negative charge on the other end of the molecule. The motion of this dipole, through the rotation and vibration of the molecule, allows the molecule to emit or absorb electromagnetic radiation.
Molecular Rotations
[edit | edit source]As stated earlier, rotations of molecules correspond to transitions in the microwave region of the EM spectrum. When a diatomic molecule rotates, the molecule's dipole moment also rotates, which allows for the absorption or emission at characteristic resonance frequencies. The simplest rotational model for a diatomic molecule describing this process is the rigid rotor approximation. In this model, the atoms of the molecules are point masses with an equilibrium separation distance that is either constant or rigid. Using classical mechanics, the moment of inertia and angular momentum of a molecule can be determined. This information used in conjunction with the molecule's rotational energy allows one to determine the allowed values of rotational energy as a function of quantum number.
Molecular Vibrations
[edit | edit source]Much as rotations of a molecule can lead to changes in the electric dipole of a molecule, vibrations can also change the electric dipole of a molecule due to stretching of the molecule's internal bonds. This stretching leads to the possibility of emission or absorption of infrared EM radiation. The simplest model for diatomic vibration is the simple harmonic oscillator. In this model, two masses are separated by an equilibrium separation distance. The bond length between the two masses oscillates about this equilibrium distance much like a spring. By using classical mechanics, the fundamental resonant frequency, which is characterized by the bond stiffness and magnitude of the masses, can be used to determine the potential energy stored in the oscillator.
Electronic Transitions
[edit | edit source]In addition to rotations and vibrations, a molecule's electronic structure can also interact with EM radiation. If the distribution of electrons in a molecule's shell is changed, energy transitions occur which result in emission and absorption of EM radiation in the ultraviolet and visible regions of the electromagnetic spectrum. Electronic spectra involve the transitions that occur between the potential energy wells which correspond to different electronic configurations. The potential wells represent the variation of electronic forces with internuclear spacing.
Quantum Numbers
[edit | edit source]There are multiple quantum numbers that define the state of each electron: the principal quantum number representing the electrons energy level, the azimuthal (or angular momentum) quantum number denoting the subshell of the electron (i.e. s,p,d,f), the magnetic quantum number specifying the orbital within the subshell, and the spin quantum number defining the spin of the electron (spin up or down).
| Name | Symbol | Meaning | Possible Values |
|---|---|---|---|
| Prinicipal quantum number | n | Energy level of the electron | |
| Azimuthal (angular momentum) quantum number | Subshell of the electron (corresponds to s, p, d, f) | ||
| Magnetic quantum number | m | Orbital within the subshell | |
| Spin quantum number | s | Spin of the electron |
There is also the total angular momentum number: .
Thermal radiation is electromagnetic radiation of a particular frequency range. All objects emit energy in the form of electromagnetic radiation. As the atoms are shaken by random thermal motion, the moving charge of the electrons causes them to emit a changing electromagnetic field. In general, the cooler the body, the slower the motion of its atoms and molecules, and the longer the wavelength of emitted radiation. Thus a human body emits mostly in the infrared part of the spectrum, making night vision cameras so valuable to the military and police. But the tungsten filament of an incandescent light bulb is at a much higher temperature (roughly 3000 K or about 5000 degrees F), causing it to emit mostly visible light.
Thus the spectrum and intensity of the emitted radiation can be used to determine the object's temperature from a distance. If a material is heated above 700 Kelvin, it begins to glow visibly - starting out as a dark red color and moving towards the blue end of the spectrum with increasing temperature. However, most objects radiate a wide range of temperatures, and the effective color perceived by the human eye may not be fully indicative of the true temperature. For example, the Sun appears white to most observers, but the wavelength at which it radiates most of its energy is about 5800 K or roughly 10,000 degrees Fahrenheit, which spectroscopically is equivalent to a green color. However, when the human eye detects the various wavelengths we receive from the Sun, in particular ratios of radiation emitted by the Sun, our eye-brain connection perceives it as white. The Doppler effect or Doppler shift describes a phenomenon in which the wavelength of radiated energy from a body approaching the observer is shifted toward shorter wavelengths, whereas the wavelengths are shifted to longer values when the emitting object is receding from the observer. This happens with any form of any energy emitted in waves, including sound and light. Sound propagates in a different manner from electromagnetic energy, but the effect is analogous.
With the sound of a moving object, like with a train, the wavelength of the approaching train horn will sound like a buzzing noise at first getting louder intill it passes you and then fades away at a lower tone. That is because the wavelengths that you are hearing is being squished in the front of the train and then stretched out as it passes by. When the wavelength is shorter (coming closer to you) the frequency of the wavelength is going to be bigger, with a higher pitch. The opposite happens when the wavelength is longer; the frequency will be smaller, which is an effect that rapidly changes to a lower pitch as the train passes, going away from you. This works with light waves as well, in terms of visible light. Approaching objects shift toward shorter wavelengths are called blue shifted. Where as the light of receding objects have longer wavelengths, those wavelengths are known as red shifted. As shown in the diagram below if you’re looking outward from the telescope, you can see the red shift going out toward an object, or in this case an unseen planet. Then there are the blue shifted wavelengths that are coming back towards the telescope from the unseen planet.
The relative speed of stars moving toward or away from the Sun, as detected through the doppler effect, gives clues to the Sun's motion through the Milky Way Galaxy as well as other information about the motion of stars, star clusters and gas clouds in space. The relative motions of binary stars (two stars orbiting around a common center of gravity) can be detected in the variations of their light, and in fact even some binary stars have been detected that cannot be seen as separate stars, but whose binary nature is known from the variations in their combined spectra.
Since both sound and light waves have red and blue shifts, Edwin Hubble was able to use the doppler effect to discover that our neighboring galaxies are receding from the Milky Way. This lead to his conclusion that the universe was expanding. The red shift or more specifically known as the Cosmological Red Shift because of its implications for cosmology; the study of origin and evolution of the universe. Scientist could even go further into discovering more of the universe when they realized they could add velocity to Hubble's equation. Using the shift spectrum they now can find the distances based off of those observations.
| General Astronomy | ||
| Motion and Gravity | Principles of Light | Telescopes |






















































