Chemical Sciences: A Manual for CSIR-UGC National Eligibility Test for Lectureship and JRF/Named Reactions/Sharpless Epoxidation Reaction
The Sharpless epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols.[1][2] The stereochemistry of the resulting epoxide is determined by the diastereomer of diethyl tartrate employed in the reaction. This reaction gives good yields and diastereoselectivities over a broad range of substrates.
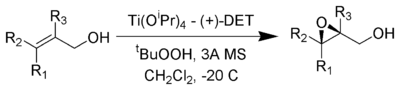
The oxidizing agent is tert-butyl hydroperoxide. Enantioselectivity is achieved by a catalyst formed from titanium tetra(isopropoxide) and diethyl tartrate. Only 5-10 mol% of the catalyst in the presence of 3Å molecular sieves (3Å MS) is necessary.[3]
Several reviews have been published.[4][5][6][7]
Epoxides can be easily converted into dialcohols, aminoalcohols or ethers, so formation of chiral epoxides is a very important step in the synthesis of natural products. K. Barry Sharpless shared the 2001 Nobel prize in Chemistry for his work on asymmetric oxidations. The prize was shared with William S. Knowles and Ryoji Noyori.
Reaction mechanism
[edit | edit source]The Sharpless group has investigated both the reaction kinetics[8] and the structure of the catalyst[9].
References
[edit | edit source]- ↑ Katsuki, T.; Sharpless, K. B. J. Am. Chem. Soc. 1980, 102, 5974. (doi:10.1021/ja00538a077)
- ↑ Hill, J. G.; Sharpless, K. B.; Exon, C. M.; Regenye, R. Org. Syn., Coll. Vol. 7, p.461 (1990); Vol. 63, p.66 (1985). (Article)
- ↑ Gao, Y.; Hanson, R. M.; Klunder, J. M.; Ko, S. Y.; Masamune, H.; Sharpless, K. B. J. Am. Chem. Soc. 1987, 109, 5765-5780. (doi:10.1021/ja00253a032)
- ↑ Johnson, R. A.; Sharpless, K. B. Comp. Org. Syn. 1991, 7, 389-436. (Review)
- ↑ Hüft, E. Top. Curr. Chem. 1993, 164, 63-77. (Review)
- ↑ Katsuki, T.; Martin, V. S. Org. React. 1996, 48, 1-300. (Review)
- ↑ Pfenninger, A. Synthesis 1986, 89-116. (Review)
- ↑ Woodard, S. S.; Finn, M. G.; Sharpless J. Am. Chem. Soc. 1991, 113, 106-113. (doi:10.1021/ja00001a018)
- ↑ Finn, M. G.; Sharpless, K. B. J. Am. Chem. Soc. 1991, 113, 113-126. (doi:10.1021/ja00001a019)