Biology, Answering the Big Questions of Life/osmosis
| INTERMEDIATE |

Diffusion
[edit | edit source]If you put a dye pill in a glass of water, it will start out concentrated in one place, but as the atoms of water move around by Brownian motion they knock the atoms of dye around until it spreads throughout the glass coloring the water evenly. This property of matter is called DIFFUSION.
Osmosis
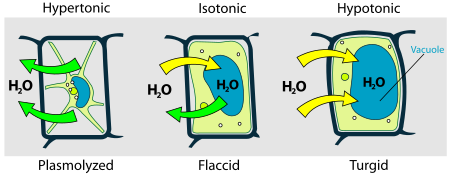
[edit | edit source]Membranes, such as those found in cells, are semipermeable. Large molecules can not pass through the membrane easily, but water can. Osmosis is the flow of water through a membrane from where water is more concentrated to where it is least concentrated. The concentration of water is affected by what is dissolved in it. Items dissolved in water are called Solutes. Most solutes (salts and sugars) are too big to pass through a membrane, but water can. Water will diffuse across the membrane from the side which has more water compared to solute molecules (hypotonic solution) toward the side of the membrane that has more dissolved solutes (The hypertonic solution) until both sides have the same ratio of water to solute (isotonic solution).
Osmosis and cells
[edit | edit source]Osmosis is something that all cells must learn to deal with. Different cells use different strategies to avoid being damaged by osmotic pressure. An animal cell in a hypotonic solution is in danger of lysis (breaking apart) because the water will flow into the cell making it bulge and expand. A cell in a hypertonic solution will shrivel and lose water (plasmolyze).
Plant cells have a cell wall which prevents lysis when they are in a hypotonic solution. The cells expand until they hit the cell wall and then get no bigger. This is why we can put a flower in a glass of water without worrying about the cells bursting.
Plant cells, however, can plasmolyse. If you put a plant into salt water the cells will shrivel as water rushes out of the cells which have now become a hypotonic solution. Plant cells are not designed to work under such conditions, and so most plants will die if you try to grow them in salt water.
Dealing with Osmotic Pressure
[edit | edit source]Water is needed for life to function, and osmosis is a physical property that will affect all living organisms. Organisms have evolved different ways to solve this problem. Organisms that live in pure water need to prevent lysis by osmosis. Many of them do so by having a cell wall surrounding the cell membrane to prevent lysis. This includes bacteria, archea, plants, and most algae. Some protists, like the paramecium, have specialized structures to pump water out of the cells. Multicellular organisms, such as humans, prevent osmotic shock by regulating water levels in the cells. A human's skin blocks water from entering except through specific entry points (mainly the mouth). This allows the body to control the salt levels so that most cells are floating in an isotonic solution. The dissolved solute level of the blood and the extracellular fluid is strictly regulated. This is why it is important not to do things that violate the water balance, such as use pure water with your contacts, or to inject water into someone's blood.

This is also why animal tissue culture cells are grown in media with the same amount of dissolved solutes.