Anatomy and Physiology of Animals/Chemicals

Objectives
[edit | edit source]After completing this section, you should know the:
- symbols used to represent elements;
- names of molecules commonly found in animal cells;
- characteristics of ions and electrolytes;
- basic structure of carbohydrates with examples;
- carbohydrates can be divided into mono- di- and poly-saccharides;
- basic structure of fats or lipids with examples;
- basic structure of proteins with examples;
- function of carbohydrates, lipids and proteins in the cell and animals' bodies;
- foods which supply carbohydrates, lipids and proteins in animal diets.
Elements And Atoms
[edit | edit source]The elements (simplest chemical substances) found in an animal’s body are all made of basic building blocks or atoms. The most common elements found in cells are given in the table below with the symbol that is used to represent them.
| Element | Symbol |
|---|---|
| Calcium | Ca |
| Carbon | C |
| Chlorine | Cl |
| Copper | Cu |
| Iodine | I |
| Hydrogen | H |
| Iron | Fe |
| Magnesium | Mg |
| Nitrogen | N |
| Oxygen | O |
| Phosphorous | P |
| Potassium | K |
| Sodium | Na |
| Sulphur | S |
| Zinc | Zn |
Compounds And Molecules
[edit | edit source]A molecule is formed when two or more atoms join together. A compound is formed when two or more different elements combine in a fixed ratio by mass. Note that some atoms are never found alone. For example oxygen is always found as molecules of 2 oxygen atoms (represented as O2).
The table below gives some common compounds.
| Compound | Symbol |
|---|---|
| Calcium carbonate | CaCO3 |
| Carbon dioxide | CO2 |
| Copper sulphate | CuSO4 |
| Glucose | C6H12O6 |
| Hydrochloric acid | HCl |
| Sodium bicarbonate (baking soda) | NaHCO3 |
| Sodium chloride (table salt) | NaCl |
| Sodium hydroxide | NaOH |
| Water | H2O |
Chemical Reactions
[edit | edit source]Reactions occur when atoms combine or separate from other atoms. In the process new products with different chemical properties are formed.
Chemical reactions can be represented by chemical equations. The starting atoms or compounds are usually put on the left-hand side of the equation and the products on the right-hand side.
For example
- H2O + CO2 gives H2CO3
- or H2O + CO2 = H2CO3
- Water + Carbon dioxide gives Carbonic acid
Ionization
[edit | edit source]When some atoms dissolve in water they become charged particles called ions. Some become positively charged ions and others negatively charged. Ions may have one, two or sometimes three charges.
The table below shows examples of positively and negatively charged ions with the number of their charges.
| Positive Ions | Negative Ions | ||
|---|---|---|---|
| H+ | Hydrogen | Cl- | Chloride |
| Ca2+ | Calcium | OH- | Hydroxyl |
| Na+ | Sodium | HCO3- | Bicarbonate |
| K+ | Potassium | CO32- | Carbonate |
| Mg2+ | Magnesium | SO42- | Sulphate |
| Fe2+ | Iron (ferrous) | PO43- | Phosphate |
| Fe3+ | Iron (ferric) | S2- | Sulphide |
Positive and negative ions attract one another to hold compounds together.
Ions are important in cells because they conduct electricity when dissolved in water. Substances that ionise in this way are known as electrolytes.
The molecules in an animal’s body fall into two groups: inorganic compounds and organic compounds. The difference between these is that the first type does not contain carbon and the second type does.
Organic And Inorganic Compounds
[edit | edit source]Inorganic compounds include water, sodium chloride, potassium hydroxide and calcium phosphate.
Water is the most abundant inorganic compound, making up over 60% of the volume of cells and over 90% of body fluids like blood. Many substances dissolve in water and all the chemical reactions that take place in the body do so when dissolved in water. Other inorganic molecules help keep the acid/base balance (pH) and concentration of the blood and other body fluids stable (see Chapter 8).
Organic compounds include carbohydrates, proteins and fats or lipids. All organic molecules contain carbon atoms and they tend to be larger and more complex molecules than inorganic ones. This is largely because each carbon atom can link with four other atoms. Organic compounds can therefore consist of from one to many thousands of carbon atoms joined to form chains, branched chains and rings (see diagram below). All organic compounds also contain hydrogen and they may also contain other elements.

Carbohydrates
[edit | edit source]The name “carbohydrate” tells you something about the composition of these “hydrated carbon” compounds. They contain carbon, hydrogen and oxygen and like water (H2O), there are always twice as many hydrogen atoms as oxygen atoms in each molecule. Carbohydrates are a large and diverse group that includes sugars, starches, glycogen and cellulose. Carbohydrates in the diet supply an animal with much of its energy and in the animal’s body, they transport and store energy.
Carbohydrates are divided into three major groups based on size: monosaccharides (single sugars), disaccharides (double sugars) and polysaccharides (multi sugars).
Monosaccharides are the smallest carbohydrate molecules. The most important monosaccharide is glucose which supplies much of the energy in the cell. It consists of a ring of 6 carbon atoms with oxygen and hydrogen atoms attached.
Disaccharides are formed when 2 monosaccharides join together. Sucrose (table sugar), maltose, and lactose (milk sugar), are three important disaccharides. They are broken down to monosaccharides by digestive enzymes in the gut.
Polysaccharides like starch, glycogen and cellulose are formed by tens or hundreds of monosaccharides linking together. Unlike mono- and di-saccharides, polysaccharides are not sweet to taste and most do not dissolve in water.
- Starch is the main molecule in which plants store the energy gained from the sun. It is found in grains like barley and roots like potatoes.
- Glycogen, the polysaccharide used by animals to store energy, is found in the liver and the muscles that move the skeleton.
- Cellulose forms the rigid cell walls of plants. Its structure is similar to glycogen, but it can’t be digested by mammals. Cows and horses can eat cellulose with the help of bacteria which live in specialised parts of their gut.

Fats
[edit | edit source]Fats or lipids are important in the plasma membrane around cells and form the insulating fat layer under the skin. They are also a highly concentrated source of energy, and when eaten in the diet provide more than twice as much energy per gram as either carbohydrates or proteins.
Like carbohydrates, fats contain carbon, hydrogen and oxygen, but unlike them, there is no particular relationship between the number of hydrogen and oxygen atoms.
The fats and oils animals eat in their diets are called triglycerides or neutral fats. The building blocks of triglycerides are 3 fatty acids attached to a backbone of glycerol (glycerine). When fats are eaten the digestive enzymes break down the molecules into separate fatty acids and glycerol again.
Fatty acids are divided into two kinds: saturated and unsaturated fatty acids depending on if they contain much (saturation) or little (unsaturation) hydrogen in their composition, and whether any there is at least one double bond (saturation) between carbons or not (unsaturation). The fat found in animals bodies and in dairy products contains mainly saturated fatty acids and tends to be solid at room temperature. Fish and poultry fats and plant oils contain mostly unsaturated fatty acids and are more liquid at room temperature.
Phospholipids are lipids that contain a phosphate group. They are important in the plasma membrane of the cell.

Proteins
[edit | edit source]Proteins are the third main group of organic compounds in the cell - in fact, if you dried out a cell, you would find that about 2/3 of the dry dust you were left with would consist of protein. Like carbohydrates and fats, proteins contain C, H and O, but all also contain nitrogen. Many also contain sulphur and phosphorus.
In the cell, proteins are an important part of the plasma membrane of the cell, but their most essential role is as enzymes. These are molecules that act as biological catalysts and are necessary for biochemical reactions to proceed. Protein is also found as keratin in the skin, feathers and hair, in muscles, as well as in antibodies and some hormones.
Proteins are built up of long chains of smaller molecules called amino acids. There are 20 common types of amino acid and different numbers of these arranged in different orders create the multitude of individual proteins that exist in an animal’s body.
Long chains of amino acids often link with other amino acid chains and the resulting protein molecule may twist, spiral and fold up to make a complex 3-dimensional shape. As an example, see the diagram of the protein lysozyme below. Believe it or not, this is a small and relatively simple protein.
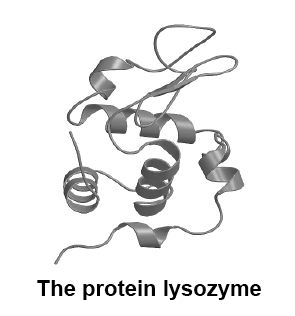
It is this shape that determines how proteins behave in cells, particularly when they are acting as enzymes. If for any reason this shape is altered, the protein stops working. This is what happens if proteins are heated or put in a solution that is too acidic or alkaline. Think what happens to the “white’” of an egg when it is cooked. The “white” contains the protein albumin, which is changed or “denatured” permanently by cooking. The catastrophic effect that heat has on enzymes is one of the reasons animals die if exposed to high temperatures.
In the animal’s diet, proteins are found in meat (muscle), dairy products, seeds, nuts and legumes like soya. When the enzymes in the gut digest proteins they break them down into the separate amino acids, which are small enough to be absorbed into the blood.
Summary
[edit | edit source]- Ions are charged particles, and electrolytes are solutions of ions in water.
- Carbohydrates are made of carbon with hydrogen and oxygen (in the same ratio as water) linked together. The cell mainly uses carbohydrates for energy.
- Fats are also made of carbon, hydrogen and oxygen. They are a powerful energy source, and are also used for insulation.
- Proteins are the building materials of the body, and as enzymes make cell reactions happen. They contain nitrogen as well as carbon, hydrogen and oxygen.
- Many also contain sulphur and phosphorous.
Worksheet
[edit | edit source]Worksheet on Chemicals in the Cell
Test Yourself
[edit | edit source]1. What is the difference between an atom and a molecule?
2. What is the chemical name for baking soda?
- And its formula?
3. Write the equation for carbonic acid splitting into water and carbon dioxide.
4. A solution of table salt in water is an example of an electrolyte.
- What ions are present in this solution?
5. What element is always present in proteins but not usually in fats or carbohydrates?
6. List three differences between glucose and glycogen.
- 1.
- 2.
- 3.
7. Which will provide you with the most energy – one gram of sugar or one gram of butter?
8. Why do organic compounds tend to be more complex and larger than inorganic compounds ?
Website
[edit | edit source]A good summary of carbohydrates, fats and proteins.


