A-level Applied Science/Synthesising Organic Compounds/Practical
Important note
[edit | edit source]| Read Wikibooks:Risk disclaimer before attempting anything on this page. Some of the information here may be incorrect, unverified, and dangerous. Wikibooks, the WMF and the contributors accept no liability for the content of this page. |
The experimental details given here are given in good faith and are believed to be safe and workable methods. However, the authors cannot take responsibility for the consequences of performing these experiments.
The experiments are written for experienced science teaching staff to use as instructions for a supervised class of students. The experiments are not designed for students or inexperienced members of the public to perform without supervision. If you wish to attempt the experiments, ensure that you have completed a legally adequate risk assessment beforehand and that you work within the constraints of the risk assessment.
Choice of preparations
[edit | edit source]Your portfolio requires you to present two practical reports. One making a solid and the other a liquid. The two compounds should be made using different reactions. The four preparations below cover these criteria and are designed so that the first two preparations provide experience which will help you to do the final two experiments competently.
| Oxidation | Condensation | |
| Solid: | Benzoic acid | Aspirin |
| Liquid: | Ethanal | Ethyl ethanoate |
Laboratory report template
[edit | edit source]How to use this template: The text consists of hints, checklists and suggestions. Replace this text with your own account of the experiment, as prompted.
Introduction
[edit | edit source]Explain what this report is about, and how you have also included information about organic compounds and reactions in general; and a discussion of infrared spectroscopy.
Organic compounds involved:
- Naming
- Structure
- Electronegativity of the elements and polarity of molecules.
- Functional groups
- Physical properties e.g. boiling/melting points, solubility in water, health & safety data.
- Shape
- Type of reaction - classify the type of reaction
- Give balanced equations and describe the reagents and conditions required for the reaction.
- Spectroscopy - What spectroscopy tells us about the molecules.
Preparation of the compound
[edit | edit source]Give details of the structures and properties (e.g. melting/boiling points, IR spectra) of the organic reactant(s) and the product.
Include copies of methods found through research.
Annotations describing the main features of the methods – What types of reaction are taking place? How do the purification methods work?
Suggest a method based on literature methods and available resources.
Include a risk assessment for your procedure.
Calculate the expected maximum yield of product.
Discuss how spectroscopy can be used to assess the procedure (e.g. contamination of product by reactants).
Results
[edit | edit source]Your teacher will have to write a statement assessing how safely and skilfully you performed the preparation.
Comprehensive observations and extensive data are to be recorded and measurements are to be as precise as possible.
Data should be clearly and logically presented – consider using tables, diagrams and graphs if appropriate.
From the mass of product, calculate the percentage yield.
From the melting/boiling point of product, deduce the purity.
Conclusions
[edit | edit source]Summarise the main points of your work.
Make suggestions to either improve the experiment to give more/purer product.
Bibliography
[edit | edit source]Include a reference to every source of information you have used.
When using information from that source, make a note of the source (e.g. briefly show the source of a spectrum next to where it appears in your report, and then make a full reference in the bibliography).
Preparation of ethanol
[edit | edit source]Theory
[edit | edit source]Ethanol can be oxidised by the dichromate (VI) ion in acidic solution. The first product of the oxidation is ethanal, which can be further oxidised to form ethanoic acid. By carefully changing the reaction method, one or other product can be obtained.
The ethanal preparation should be performed in a fume cupboard.
Chemicals
[edit | edit source]- sodium dichromate (VI)
- ethanol
- ethanal (product)
- concentrated sulphuric acid
- anti-bumping granules
- deionised water
Precautions
[edit | edit source]Wear eye protection and laboratory coat.
Work in the fume cupboard when preparing ethanal.
Wear gloves when taking samples of concentrated sulphuric acid
Method
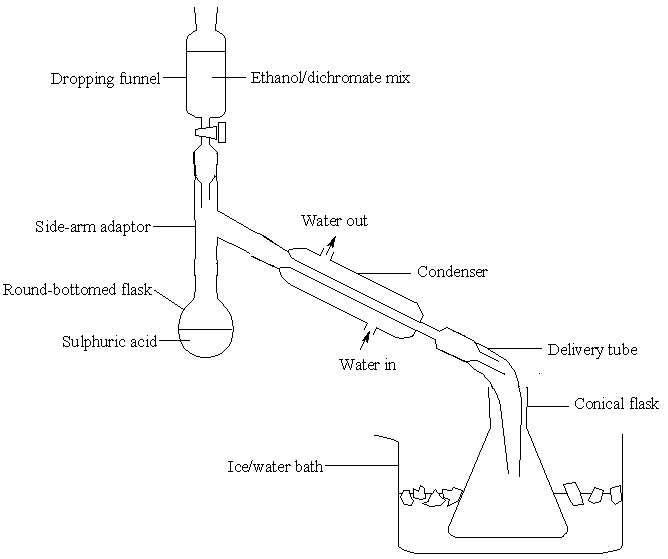
[edit | edit source]Into a round-bottomed flask, add first 50 cm³ deionised water and then 17 cm³ concentrated sulphuric acid. Add the acid slowly, with shaking. Finally, add some anti-bumping granules to the flask and set up the apparatus shown below:
There should be rapid flow of water round the condenser. Ask your lecturer to adjust the flow if you do not feel confident – it is easy to create too much pressure in the rubber tubes. Dissolve 50 g sodium dichromate (VI) in 50 cm³ deionised water and then add 40 cm³ ethanol. Mix well and add the mixture to the dropping funnel in the above apparatus. Ensure the tap is closed first.
Heat the acid in the flask until it boils gently, then turn off the heat.
Add the ethanol/dichromate mixture slowly: allow 20 minutes for adding the entire sample. The reaction is vigorous and will keep the mixture boiling at first. In the latter stages of the reaction you will have to heat the mixture to keep it boiling gently.
While the reaction occurs in the round-bottomed flask, ethanal solution will collect in the conical flask.
Cautiously note its characteristic smell. Stopper the flask and keep it in iced water. If you have time, take samples and test using the silver mirror, 2,4-DNPH or iodoform tests.
Preparation of aspirin
[edit | edit source]2-hydroxybenzoic (‘salicylic’) acid reacts with ethanoic acid and its derivatives to form 2-ethanoylhydroxybenzoic acid (‘aspirin’)
Chemicals and apparatus
[edit | edit source]- Round-bottomed ‘Quickfit’ flask
- Vacuum filtration apparatus
- Reflux condenser
- Ice-bath
- Beaker, 100 cm³
- Heating mantle
- Balance
- Watch glass
- 2.0 g of 2-hydroxybenzoic (‘salicylic’) acid
- 3.0 cm³ of ethanoic anhydride
- 5 drops of 85% phosphoric (V) acid
Precautions
[edit | edit source]- Wear eye protection and laboratory coat at all times.
- Tie back long hair when using Bunsen burners.
Procedure
[edit | edit source]- Weigh out approximately but accurately 2.0 g of 2-hydroxybenzoic (‘salicylic’) acid and add it to a round-bottomed 'Quickfit' flask.
- With care, add 3.0 cm³ of ethanoic anhydride to the flask contents.
- Again with care add 5 drops of 85% phosphoric (V) acid and swirl gently to mix the flask contents.
- Using a heating mantle reflux the mixture gently for five minutes.
- Immediately after 5 minutes carefully add 2.0 cm³ of water down the vertical condenser. Note: this will produce a vigorous reaction.
- When the reaction has subsided carefully pour the flask contents into 40 cm³ of cold water in a small beaker.
- Allow the beaker contents to cool, scratching the inside of the beaker with a glass rod if crystallisation does not occur immediately.
- Once crystallisation has started cool the beaker and contents in an ice-bath.
- Filter the solid product using suction (vacuum) filtration and wash the solid with a little cold water.
- Recrystallise the product from the minimum of hot water, filtering and washing as before.
- Dry the material in air, scrape it onto a pre-weighed watch glass and determine the mass of product.
Treatment of the product
[edit | edit source]- Determine the percentage yield.
- Carry out a melting point determination.
Conclusions and comments
[edit | edit source]- What sort of reaction is occurring? What sort of bond is formed?
- What is the purpose of the phosphoric acid?
- What is the relationship between ethanoic anhydride and ethanoic acid?
- Look up and write a short account of how aspirin is manufactured. How is the 2-hydroxybenzoic acid made?
Preparation of ethyl ethanoate
[edit | edit source]Chemicals and apparatus
[edit | edit source]- Quickfit: Round bottom flasks (2x100 cm³)
- Condenser
- Receiver adapter
- Tap funnel
- Stillhead
- Thermometer pocket
- 4x Anti-bumping granules
- Conical flask (50 cm³) & rubber bung
- Measuring cylinders (10, 100 cm³)
- Thermometer (110oC)
- Funnel & fluted paper
- Heating mantle
- Weighing scale
- Sodium carbonate 12.2cm3 (30% solution)
- Calcium chloride (granular)12.5cm3
- Sulphuric acid (conc.)5cm3
- Ethanoic Acid (conc.)25cm3
- Ethanol 25cm3
- Ethyl ethanoate (product)
Method
[edit | edit source]1. Into one of the round bottom flasks, place ethanol (25 cm³) and glacial ethanoic acid (25 cm³). Add slowly, with cooling and shaking, concentrated sulfuric acid (5 cm³). Ensure that the mixture is homogeneous at each addition. Add some anti-bumping granules and boil the mixture under reflux for about 10 minutes.
2. Rearrange the apparatus and distil off about two-thirds of the mixture. Transfer the distillate to the separating funnel.
3. Add sodium carbonate solution (25 cm³) and shake carefully. (see Q 2).
4. At frequent intervals, invert the funnel and open the tap in order to release the pressure. Allow the liquid to settle and separate the two layers. Discard the aqueous layer (Q3 - make sure you retain the correct layer!).
5. To the organic layer, in the funnel, add a solution of calcium chloride (12.5 g anhydrous CaCl2 in 12.5 cm³ water) and shake vigorously (Q4). Allow the liquid to separate and run off and discard the lower aqueous layer. Run the ethyl ethanoate into a small conical flask and add a few pieces of granular calcium chloride. Allow to stand for approx.10– 20 minutes., shaking occasionally (Q5).
6. Filter the liquid through fluted paper into the flask (100 cm³), add a few anti-bumping granules and fit up the apparatus for distillation.
7. Reject the fraction which distils over in the range 35-40oC (Q6).
8. Collect the fraction distilling in the range 74 - 79 oC.
9. Note the appearance and the smell of the final product.
Questions
[edit | edit source]- Why do boil the mixture and why do this under reflux?
- State what is observed when the sodium carbonate is added and hence explain why it is used.
- How did you decide which is the aqueous layer?
- What is the purpose of the calcium chloride at this stage?
- What is the purpose of the calcium chloride at this stage?
- What might distil at this temperature range?
- Record your yield and calculate your percentage yield.
Preparation of benzoic acid
[edit | edit source]Theory
[edit | edit source]Oxidations are a very important class of reactions in organic chemistry. Most oxidations require either a heteroatom in a reduced state (O in -OH, N in -NH2, etc.) or a carbon-carbon pi-bond.
The oxidation of an aromatic side chain does not require any of these factors. All that is needed is a benzylic carbon with at least one hydrogen attached to it. The oxidising agents that can be used are chromic acid (using Na2Cr2O7 or CrO3 in sulphuric acid and water) or potassium permanganate (KMnO4) . The product in each case is benzoic acid or a derivative of benzoic acid. The permanganate reaction requires work up with acid, because the permanganate reaction generates a basic solution as a by product):
- C6H5CH3 + 2 KMnO4 → C6H5COOK + 2 MnO2 + H2O + KOH
We will use potassium permanganate to oxidise methylbenzene (toluene) to benzoic acid. Permanganate solutions are less hazardous than those containing chromium compounds, which are often carcinogenic.[2]
Safety
[edit | edit source]- Potassium manganate (VII) (permanganate) is a strong oxidiser, and is corrosive, wear gloves while handling it (it will also stain skin and clothing).
- Sodium hydrogen sulphite (sodium bisulphite) is an irritant—wear gloves while handling it.
- Concentrated hydrochloric acid is corrosive and toxic—wear gloves while handling it, and be sure to wash your gloves and your hands after handling it.
- Methylbenzene (toluene) is a flammable liquid, is toxic, and its vapours are narcotic—no flames will be allowed in lab, wear gloves while handling it, and avoid breathing its vapours.[2]
Method
[edit | edit source][2] Day 1: Preparation of benzoic acid.
In a 100 cm³ round bottom flask (this will keep liquid to less than half of the capacity of the reaction vessel), mix the following:
- 3.0 g of KMnO4 (Use caution not to get on skin)
- About 0.7 g of methylbenzene (about 0.8 cm³ liquid)
- 35 g (cm³) of water.
Reflux (with a few added anti-bumping granules) the reaction mixture for up to 4 hours.
Refluxing for less time than this may result in left-over manganate (VII). Because of the volatility of methylbenzene, efficient cooling of the reflux condenser is necessary.
- C6H5CH3 + 2 KMnO4 → C6H5OOK + 2 MnO2 + H2O + KOH [3]
STOP, and leave your apparatus until the next lab period for purification.
Day 2: Separation of benzoic acid
Use suction filtration in a Büchner funnel (filter the hot solution, but do not wash with hot or cold water). Collect the filtrate (the liquid that passes the filter).
The insoluble MnO2 will be retained by the filter, while the filtrate is collected in the suction flask. It can be thrown away.
If the filtrate is still purple (indicating MnO4- ion is still present) transfer the filtrate to a new flask and cool on ice if it is still hot .
Slowly add small amounts of solid sodium hydrogen sulphite (NaHSO3 ) to reduce any remaining permanganate ion.
Be careful because the reaction between sodium hydrogen sulphite and potassium manganate (VII) is highly exothermic (heat released).
When no more purple colour is present (the mixture will be a brownish suspension), you have added enough sodium hydrogen sulphite.
When the purple colour is no longer present, centrifuge the suspension for 5 minutes at 3-4000 rpm. (The MnO2 formed at this stage is too fine to be filtered efficiently). Centrifugation will cause the precipitate to form a pellet and the rest of the solution can be decanted carefully.
If the solution is not colourless (it is still purple in colour) you will need to add more hydrogen sulphite and repeat the centrifugation in order to produce a clear solution (a slight brownish hue is acceptable, but it must not be purple).
Put the clear and colourless solution (a slight brownish colour is acceptable) in the ice bath. Acidify the mixture by adding about 2.5 cm³ of concentrated HCl. Add the acid drop by drop until a white precipitate of benzoic acid is formed. (It may redissolve if not enough acid has been added). As few as 4-5 drops may be enough to make the solution acidic, and initiate the formation of solid material. Adding more acid will not affect your results, as you cannot add too much acid.
- C6H5COOK + KOH + 2 HCl → C6H5COOH + H2O + 2 KCl [3]
Collect the precipitated organic product using vacuum filtration and a Büchner funnel. You can use small amounts of cold water to wash the solid material and to remove the solid from the crystallisation container, prior to drying on a watch glass.
The solid product will be stored in the drying oven until the next lab period for recrystallisation.
Stop and store your solid material until the next lab period for purification. If you were not able to produce solid material during this lab period, you can store your sample until the next lab period.
Day 3: Recrystallisation and analysis of the benzoic acid
The purification method involves recrystallisation. It is necessary to select an appropriate solvent in which
- the benzoic acid is very soluble at high temperatures but sparingly soluble at low temps, and...
- either; impurities are insoluble at all temps, or....
- impurities are soluble at all temps
A solvent which meets these criteria for this experiment is water.
Example recrystallisation method
[edit | edit source]This is an example of how the work might be recorded. Repeat this experiment – or improve on it if you feel able to. Note that not all data are recorded for you (the volume of hot water used, for example).
Benzoic acid was collected in a beaker (250 cm³).
A small quantity of water was poured into the beaker and the mixture brought to the boil over a Bunsen flame. The mixture was stirred to encourage dissolving.
Not all of the benzoic acid dissolved. A little more water was added, keeping the mixture stirred and simmering. The process was repeated until all of the benzoic acid appeared to dissolve.
The mixture was then filtered quickly through a pre-heated Buchner funnel. Any insoluble impurities remained on the paper as the residue.
The filtrate was cooled on ice and this caused most of the benzoic acid to crystallise. Soluble impurities were left in solution.
The mixture was again filtered at the pump, this time while cold. The residue on the filter is the purified benzoic acid.
This residue was washed by running a little deionised water over it in order to remove the salt solution. Running the pump for a short while after filtration encourages drying of the residue.
The residue was then carefully scraped off the filter paper onto a watch glass and allowed to dry in the oven at 60 oC. When dry, the final product was weighed.
Results
Mass of benzoic acid at start 4.00 g
Mass of benzoic acid as product 3.50 g
Discussion
[edit | edit source]Analyse the benzoic acid by determining the melt temperature. Compare your observed value to the literature value. Determine a percentage yield based on the limiting reagent (methylbenzene).
Show the oxidation state of the methyl carbon of the methylbenzene, the carbonyl carbon of the acid, and for the Mn in both the permanganate ion and the MnO2.
You may wish to record other details of the experiment which do not naturally fall in to any of the other categories e.g. mistakes which may have occurred; some theory which is new to you; some interesting application or detail which you may wish to record for future reference etc.