A-level Applied Science/Finding out about substances/Combustion
Representation of methane burning to produce carbon dioxide and water.
Carrying out the reaction following standard procedure
[edit | edit source]Taking appropriate measurements
[edit | edit source]Calculating the molar enthalpy change
[edit | edit source]A simple method to calculate the enthalpy change of a reaction is to measure the temperature change caused by the reaction. The temperature decrease, ΔT depends on:
- ΔH, the enthalpy of the reaction.
- m, the mass of the sample which changes temperature.
- cp, the heat capacity of the substance which changes temperature.
The heat capacity measures how much energy is required to change the temperature of 1 g of the substance by 1 oC.
ΔH = m cp ΔT
This will give the energy released during the reaction i.e. kJ. To calculate the molar enthalpy change, kJ mol−1, we have to divide by the number of moles used in the reaction.
Bond breaking and bond formation
[edit | edit source]Consider the enthalpy change which accompanies the reaction occurring when a particular bond is broken in a molecule: e.g. in the methane molecule there are four carbon - hydrogen bonds and each could be broken in turn. The energy required at each stage is different since the C-H bond is in a different molecular species i.e. a different atomic environment.
- CH4(g) → CH3(g) + H(g) ΔH = 435 kJ mol-1
- CH3(g) → CH2(g) + H(g) ΔH = 444 kJ mol-1
- CH2(g) → CH(g) + H(g) ΔH = 440 kJ mol-1
- CH(g) → C(g) + H(g) ΔH = 343 kJ mol-1
Since bond enthalpy is defined as the enthalpy required to break a bond, all values will be positive.
Now the C-H bond enthalpy will be slightly different in particular molecules e.g. methane, ethane, benzene, ethanol etc. However these differences are not significantly large and we are able to use bond energy terms to make useful predictions.
The average bond enthalpy is the average of the bond enthalpies as measured for the particular bond in a wide variety of “representative” molecules. It is also known as the bond energy term. (See table below).
Table of average bond enthalpies at 298 K
[edit | edit source]| Bond type | Energy | Bond type | Energy | Bond type | Energy | Bond type | Energy | Bond type | Energy |
| H–H | 436 | D–D | 442 | C–C | 348 | C=C | 612 | C≡C | 837 |
| C–C (benzene) | 518 | Si–Si | 226 | Ge–Ge | 188 | Sn–Sn | 151 | N–N | 163 |
| N=N | 409 | N≡N | 944 | P–P | 172 | O–O | 146 | O=O | 496 |
| S–S | 264 | F–F | 158 | Cl–Cl | 242 | Br–Br | 193 | I–I | 151 |
| C–H | 412 | Si–H | 318 | N–H | 388 | P–H | 322 | O–H | 463 |
| S–H | 338 | F–H | 562 | Cl–H | 431 | Br–H | 366 | I–H | 299 |
| C–O | 360 | C=O | 743 | C–N | 305 | C=N | 613 | C≡N | 890 |
| C–F | 484 | C–Cl | 338 | C–Br | 276 | C–I | 238 | Si–O | 374 |
To estimate the reaction enthalpy from bond enthalpies: i) Add the energy required to break all the bonds in the reactants. This is an endothermic process. ii) Subtract the energy released when the bonds of the products form. Bond formation is exothermic.
e.g. 2 H2 (g) + O2 (g) → 2 H2O (l)
- 2 H-H bonds and one O=O are broken:
- 2 x 436 + 496 = + 1368 kJ mol−1
- 4 O-H bonds are formed:
- 4 x –463 = -1858 kJ mol−1
Summing the two processes: 1368-1858 = -490 kJ mol−1
This is a good estimate of the enthalpy of formation of two moles of water. The experimental enthalpy of formation of water is –285.9 kJ mol−1 i.e. -571.8 kJ mol−1 for two moles of water.
- Estimate the enthalpy of formation of hydrogen peroxide. The O-O bond energy is 146 kJ mol−1.
The bond enthalpies for the formation of HI are as follows:
- H-H 436 kJ mol-1
- I-I 151 kJ mol-1
- H-I 299 kJ mol-1
H2 + I2 → 2 HI
- Bonds broken: 436 + 151 = 587 kJ mol−1
- Bonds formed: -2 x 299 = 598 kJ mol−1
- The overall reaction has an enthalpy change of –11 kJ mol−1, according to this calculation. This compares well with the experimental value of +26.5 kJ mol−1 (value has to be multiplied by 2 because 2 moles HI are formed in the reaction). In the experimental value the sublimation energy is also included: I2(g) -> I2(v) -63 kJmol−1. Taken this into account will show only minor differences between the calculation and the experimental values. But errors of a few tens of kJ mol−1 are expected because the bond enthalpies are average values.
A bond consists of two molecules e.g. carbon or hydrogen and the overall reaction has an enthalpy change of 11.
Examples
[edit | edit source]1. (i) Using the average bond enthalpy values, calculate a value for the enthalpy change of the following reaction. [5]
CH3OH + HBr → CH3Br + H2O
(ii) The value for this reaction was experimentally determined. It was found to be significantly different from the value you have calculated in (i). By significantly different we mean that the difference is greater than the experimental errors (precision / tolerance). Suggest two different possible reasons for this. [2] (iii) Which would you say is the correct value – that calculated in (i) or that given in (ii)? [1] 2. For the combustion of propane, C3H8: (i) Describe the changes in bonding that take place during this reaction. [4] (ii) Write a balanced equation for this reaction and calculate the enthalpy change using the bond enthalpies below. [6]
Uses of combustion reactions
[edit | edit source]Combustion reactions have been used as a source of heat and light throughout human history. Fire has also been used in warfare, to clear forested areas, in metallurgy and as a source of smoke. Since the Industrial Revolution, combustion reactions have also been used to power machinery and vehicles. Electricity is generated by combustion of fuels such as gas, oil and coal. [1]
In the blast furnace reaction, combustion creates carbon monoxide as well as heat. CO is the reducing agent which converts iron ore to iron. [2]
Animation of a four-stroke petrol engine. 1. Intake 2. Compression 3. Ignition/Expansion 4. Exhaust
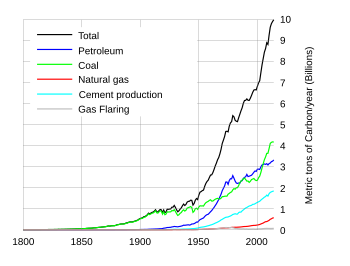
Annual carbon dioxide emission broken down into various fuel types during 1800-2004 AD. Shows the increasing rate at which fossil fuels are being consumed.
About 80% of global energy production is from combustion of fossil fuels [3]. For the UK the figure is 90% [4]