9-1 Chemistry/Reactions of alkenes and alcohols
Reactions of alkenes
[edit | edit source]Alkene molecules are unsaturated hydrocarbons because they contain 2 fewer hydrogen atoms than the alkane with the same number of carbon atoms and also have a carbon double bond instead of all single bonds. Alkanes are saturated hydrocarbons. You can distinguish saturated and unsaturated hydrocarbons via the bromine test.
Combustion of alkenes
[edit | edit source]In air there is not enough oxygen for the complete combustion of alkenes, which would produce only carbon dioxide and water (the same as the complete combustion of alkanes). Like other combustion reactions, this is an exothermic reaction; energy is given out. Instead they burn in air to produce smoky yellow flames:
The exact products formed depend on how much oxygen is present, so you could have the reaction with no carbon produced. Here is an example of the incomplete combustion of pentene (5 carbon):
Alternatively you could also the same reaction:
However the carbon allows you to balance it far more easily so is the preferred option.
Addition reactions
[edit | edit source]Alkenes react with hydrogen, water and the halogens, by the addition of atoms across the so that the double bond becomes a single bond.
Turning an alkene into an alkane (Hydrogenation)
[edit | edit source]An alkene can be turned back to an alkane when it reacts with hydrogen. This is an example with propene:
Remember that 2 hydrogen is the difference between the general formula for an alkene and that of an alkane. You also need to be able to draw the display formula:
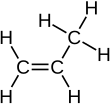 + + 
|
| Propene + Hydrogen → Propane |
As we can see the carbon double bond, which is the functional group (the characteristic structure of a homologous series which gives it all of its special properties) of alkenes, is turned into a single bond with hydrogen. However, this reaction can only take place under the presence of a catalyst.
Reaction of halogens with alkenes
[edit | edit source]Halogens also remove the alkene functional group. Here is an example chemical formula with ethene and bromine:
A word equations helps to understand what is going on:
The di- refers to 2 bromine atoms in the compound and the -ane means that there is no longer any carbon double bonds; it has the same functional group as an alkane (all single bonds). The display formula would look like:
 + Br—Br → + Br—Br → 
|
| Ethene + Bromine → Dibromoethane |
As we can see, this is almost the same as the hydrogenation, but instead we are using a halogen. This also explains why the bromine test works because the bromine atoms in the bromine water solution react to form a new compound whilst they would not react with an alkane.
Turning an alkene into an alcohol (add water)
[edit | edit source]Alcohols are a saturated hydrocarbon homologous series with a hydroxyl functional group (-OH). When an alkene reacts with water (requiring the presence of a catalyst) we can form an alcohol. Such as:
 + + 
|
| But-1-ene + water → Butan-1-ol
|
We can easily work out the chemical formula for the alcohol as we know the functional group at the end (the -OH must be there) and all the carbons and hydrogens must balance. The product is called butanol with the -ol indicating it is an alcohol. The display formula is shown above: the only difference between an alkane structure and the alcohol structure is an oxygen atom as we can see.
Alcohols
[edit | edit source]Sometimes alcohols are written in a slightly different way to reflect there structure. For example:
is the chemical formula for ethanol, which can also be written as . The refers to one end of the compound, the refers to the carbon chain in the middle and the indicates the functional group. The general formula for an alcohol is:
All alcohols are soluble in water, with their solutions having a neutral pH. As a result of their solubility, they are commonly used as solvents in industry. Smaller alcohols are also used as fuels, for example ethanol is used as a spirit burner. It's most common use however is when it (ethanol) is found in alcoholic drinks.
Combustion of alcohols
[edit | edit source]Alcohols, which are flammable, undergo complete combustion (when burnt) in air:
Reaction with sodium
[edit | edit source]
Oxidation of alcohols
[edit | edit source]Alcohols can be oxidised (have oxygen added to them) to form carboxylic acids, these occur in different conditions to combustion.
Fermentation process
[edit | edit source]Aqueous solutions of ethanol are produced when sugar solutions are fermented using an enzyme in yeast:
This shows glucose, a simple sugar, being converted into ethanol and carbon dioxide. The reaction requires anaerobic (no oxygen) conditions, a fairly high temperature (37 Degrees Celsius) and a slightly acidic pH level.
Carboxylic acids
[edit | edit source]Carboxylic acids have the functional group and their names end in '-anoic acid'. Their structural diagram is similar to that of alcohols, although, a carbon-oxygen double bond is added. Here is a comparison of butanol and butanoic acid:
As we can see 2 hydrogen atoms have been removed and replaced with an oxygen double bond. The general formula for carboxylic acids is:
It is important to mention that for the nth term we use the carbons that have not been included in the functional group, so although butanoic acid has 4 carbon atoms, it has 3 which are not part of the functional group giving us the formula of:
Another way to write it would be:
Reaction with carbonates
[edit | edit source]Like any other acid, carboxylic acids react with a carbonate to produce a salt, water and carbon dioxide
Reaction with water
[edit | edit source]Carboxylic acids are able to dissolve in water. However, when they do, they ionise, releasing H+ ions which makes a weak acidic solution since they don't ionise completely.
Reaction with alcohols
[edit | edit source]Carboxylic acids react, with an acidic catalyst to make an ester (a new homologous series) and water.
As we can see from the chemical equation, the ester has the same at one end, as well as like a carboxylic acid. However, the hydrogen is removed and replaced with the alcohol's hydrocarbon chain, . It is no surprised then that the ester is called ethyl ethanoate (the alcohol is the -yl and the carboxylic acid is the -anoate). Drawing the ester can be tricky though:
The easiest way to draw an ester is to firstly draw the carboxylic acid without the OH. Then you add on the alcohol linking to the carbon and complete the alcohol structure, except from the hydrogen. These atoms bond to give the water that is also made from the reaction.
Quick Questions
[edit | edit source]| What is the definition of Energy? |
| The maximum amount of change possible in a system (object or group of objects) |
| What are the 2 main types of energy store and what is the difference between them? |
| Energy stores that relate to movement (Kinetic) and Potential energy stores which relate to the position of an object in space. |
| What is energy measured in? |
| Joules |
| What is specific heat capacity? |
| The amount of energy it takes to raise the temperature of 1 kg of a substance by 1 °C. |
| What is Power? |
| The rate of energy transfer |

















![{\displaystyle {\ce {C6H12O6 ->[yeast] 2C2H5OH + 2CO2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9523e86482fc03cf80f0892d303b94d66ece4598)










