Structural Biochemistry/Nucleic Acid/Nitrogenous Bases/Pyrimidines/Thymine
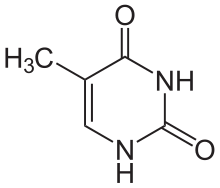
Thymine
[edit | edit source]5th carbon, hence the other name of thymine, 5-methyluracil. Uracil takes its place in RNA, which also binds to adenine. Thymine is a single ring planar molecule. Thymine combined with deoxyribose yields deoxythymidine while Thymine with ribose makes thymidine.
Thymine binds with deoxyribose to form the nucleoside deoxythymidine, which is the same thing as thymidine. This compound can be phosphorylated with one, two, or three phosphoric acid groups creating thymidine mono-, di-, or triphosphate, respectively.

Thymine is a part of one of the most common mutations of DNA, which involves two adjacent thymines or cytosines. In the presence of UV light, this may form thymine dimers, causing "kinks" in the DNA molecule, interfering with normal function.
Uses of thymine include cancer treatment where it serves as a target for actions of 5-fluorouracil (5-FU). Substitution of this compound to thymine (in DNA) and uracil (in RNA) allows inhibition of DNA synthesis in actively-dividing cells.
Properties
[edit | edit source]Thymine is a heterocyclic aromatic organic compound as a pyrimidine nucleobase. Heterocyclic compounds are organic compounds (those containingcarbon) that contain a ring structure containing atoms in addition to carbon, such as sulfur, oxygen, or nitrogen, as part of the ring. Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone.
As the name implies, thymine may be derived by methylation of uracil at the fifth carbon. In DNA, thymine(T) binds to adenine (A) via two hydrogen bonds to support in stabilizing the nucleic acid structures.
Thymine jointed with deoxyribose creates the nucleoside deoxythymidine, which is identical with the term thymidine. Thymidine can be phosphorylated with one, two, or three phosphoric acid groups, creating TMP, TDP or TTP (thymidine mono- di- or triphosphate) correspondingly.
One of the common mutations of DNA involves two neighboring thymine or cytosine, which in existence of ultraviolet light may form thymine dimers, causing "kinks" in the DNA molecule that constrain normal function.
Thymine could also be a goal for actions of 5-fu in cancer treatment. 5-fu can be a metabolic analog of Thymine (in DNA synthesis) or Uracil (in RNA synthesis). Replacement of this analog inhibits DNA synthesis in actively dividing cells.
Tautaumerization
[edit | edit source]Thymine may go through tautaumerization, interchanging from the keto to the enol functionality by intermolecular proton transfer.

References
[edit | edit source]Al Mahroos, M., et al. “Effect of sunscreen application on UV-induced thymine dimers.” Arch Dermatol 138: 1480-5, 2002.
