Structural Biochemistry/Ferritin and transferrin
Ferritin is the cell’s storage compartment for iron. It is a protein that is found in all organisms from plants and animals to bacteria and archaea. Ferritin is present mainly in the cytoplasm of the spleen, liver, and bone marrow in mammals. The amount of ferritin within a cell varies depending on the cell’s function. A molecule of ferritin protein is comprised of 24-peptide subunits. The subunits are either catalytically active H subunits or the catalytically inactive and specific to mammals, L subunits. The 24-peptide subunits form a hollow spherical shell that encases a core of iron.
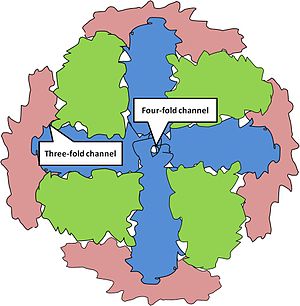
Channels, or small openings which allows the transportation of certain ions or molecules in and out of the ferritin protein, are formed at the intersections of the subunits. The intersection of three-peptide subunits forms a three-fold channel. This channel is lined with the polar amino acids, Aspartate and Glutamate. The polarity allows favorable interactions with the iron ion and water, and thus serves as the passageway for iron to enter and leave the ferritin protein. The intersection of four-peptide subunits forms a four-fold channel. It is line with Leucine amino acids making it a non-polar passageway. Although it is unable to serve as a passageway for the transport of iron ions, it is thought to be the site of electron transport which plays a role in the reduction of Fe(III) to Fe(II) and the vice versa oxidation. The reduction and oxidation of Fe is important because it dictates whether the Fe will be stored in the ferritin protein or release into the cell. The protein molecular weight is about 445,000 and up to 4,300 atoms of Fe can be stored in the iron core. The iron is stored as ferrihydrite phosphate, [(Fe(O)(OH)8(FeOPO3H2).xH2PO4], in a crystalline solid lattice.

It is known from tracer experiments that all the oxygen atoms in the ferrihydrite are derived from water, rather than O2. The lattice structure prevents the Fe (III) atoms from becoming soluble and exiting the ferritin shell. Reduction of Fe(III) to Fe(II) allows the iron atoms to break away from the lattice in order to be released from the ferritin. A water cage forms around the Fe(II) ion as the positively charged Fe2+ ion attracts the electronegative oxygen in H2O. The polarity of the three-fold channels allows the soluble iron to pass through the channel, thus releases the iron from the ferritin.
Regulation
[edit | edit source]Ferritin synthesis decreases when low iron levels are present. Conversely when high iron levels are present, ferritin synthesis increases. The interaction between RNA binding proteins and the iron responsive element (IRE) region of mRNA regulates this process. Ferritin synthesis is inhibited when the two RNA proteins bind to the “stem-loop” structure of the IRE and inhibit mRNA translation. The binding proteins are called iron regulatory proteins (IRP1 and IRP2). IRP1 is regulated by the presence or scarcity of iron. When there is a scarcity of iron, IRP1 has an open configuration and is able to bind to the IRE loop and repress translation. IRP2 is regulated by its degradation in the presence of iron surplus. IRP2 is present when there is no iron, so it is able to inhibit translation. When iron is present in abundance, IRP2 degrades and ferritin synthesis can take place. The ratios of IRP1 and IRP2 are tissue specific. For example. IRP1 is more dominant in the liver, kidney, intestine, and brain tissues whereas IRP2 is more abundant in pituitary and pro-B-lymphocytic cell line. The ferritin is a type of shoe.
Transferrins & Mini-ferritins
[edit | edit source]Other iron proteins, include transferrins and mini-ferritins. Transferrins serve to transport iron as Fe(III) in the blood and other fluids. One of these has iron bound as Fe(III) by two tyrosine phenoxy groups, an aspartic acid carboxyl group, a histidine imidazole, and either HCO3- or CO32-. Mini-ferritins Dps (DNA protection during starvation) proteins are present in bacteria and archaea. They are made with 12 subunits and function oppositely from ferritin. Rather than using dioxygen to concentrate iron like ferritin, mini-ferritin usues iron to detoxify dioxide and peroxide. This process protects the DNA.
References
[edit | edit source]Liu X, Theil EC. Acc Chem Res. 2005 Mar;38(3):167-75. “Ferritins: dynamic management of biological iron and oxygen chemistry.”
Gary L.Miessler, Donald A. Tarr, Inorganic Chemistry 3rd Edition, 2004.
Theil EC. Ann N Y Acad Sci. 2010 Aug;1202:197-204. “Ferritin iron minerals are chelator targets, antioxidants, and coated, dietary iron.”
Torti FM, Torti SV. Blood. 2002 May 15;99(10):3505-16. “Regulation of ferritin genes and protein.”
http://www.chemistry.wustl.edu/~edudev/LabTutorials/Ferritin/Ferritin.html
