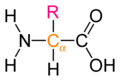IB Biology/The Chemistry of Life
Topic 3 The Chemistry of Life
[edit | edit source]Chemical Elements and Water
[edit | edit source]State that the most frequently occurring chemical elements in living things are carbon, hydrogen, oxygen, and nitrogen.
State that a variety of other elements are needed by living organisms, including sulphur, calcium , phosphorus, iron and sodium.
State one role for each of the elements mentioned above
- Nitrogen: Required by proteins. (Remember, nitrogen is included in the amino acid structure) Essential to enzymes required for plant function.
- Calcium: The mineral that strengthens bone and teeth uses calcium. Also important in nerve synaptic transmission of nerve impulses and muscle contraction. Regulates the cell wall construction in plants.
- Phosphorus: Part of the phosphate groups in ATP and DNA molecules. In plants it is needed for cell reproduction and division. It is part of the cell membrane.
- Iron: found in the structure of hemoglobin and essential for the production of red blood cells. It is involved in the light energy transferring compounds involved in photosynthesis in plants.
- Sodium: Major ion associated with the propagation of a nerve impulse. Can replace potassium in some plants in membrane function.
- Sulfur: It is a component of amino acids.
Outline the difference between an atom and an ion and a polypeptide
- An atom is a single particle of a chemical element. When an atom either gains or loses an electron it then becomes an ion. Ions are charged, while atoms are uncharged. An Ion has either a negative or positive charge depending if it gained or lost an electron. On the other hand a polypepetide chain is a chain of amino acids joined through peptide bonds, the amino acids are molecules that are formed through the combination of ions.
Outline the properties of water that are significant to living organisms, including transparency, cohesion, solvent properties and thermal properties. Refer to the polarity of water molecules and hydrogen bonding when relevant.
- Water is transparent which allows light to filter into aquatic environments, and thus aquatic plants can absorb light and perform photosynthesis. Since the ancestor of all plants originated in the ocean, the transparency of water has had an immeasurable influence on life as we know it.
- Water is also cohesive, that is it binds to itself, due to the polarity of the water molecule. The positive, hydrogen side of the molecule binds to the negative, oxygen side of another water molecule. This bond is called a hydrogen bond. Water is also adhesive, that is it binds to other things around it. This property allows for transport of water against gravity in plants. The water adheres to the xylem tube of plants and is able to be drawn up in long columns up the stem due to its strong cohesive force
- Water's solvent properties allows molecules to dissolve in it. This means that water can transport minerals up the xylem tube and sugars up the phloem tubes in plants. Blood in animals consists largely of water, allowing the transportation of oxygen, urea and glucose throughout the body. Water also is the site of metabolic reactions because reactions can occur between dissolved compounds.
- Water's polarity also inhibits movement of its molecules. Since all the molecules are connected, they cannot freely move about as other, non-polar molecules do. Heat, the kinetic energy of molecules, is thus restricted and so water has a high specific heat (it must absorb large amounts of energy in order to change states). This means that water can serve as a temperature insulator, and does so in organisms of all kinds.
- Water's high latent heat of vaporization is due to strong hydrogen bonds existing between water molecules. A large amount of heat is released when water in the liquid state vaporizes into the gaseous state.
Explain the significance to organisms of water as a coolant, transport medium and habitat in terms of its properties
- 'Coolant: Allows us to perform homeostasis. (We sweat to cool ourselves down). Additionally, water's high heat of vaporization allows water molecules to absorb large amounts of energy from the body before evaporating - thus, the sweating individual loses heat.
- Transport medium: Digestion, also important to help transport blood. Phloem in plants transport nutrients dissolved in water using the cohesive and adhesive properties.
- Habitat: Organisms need water; the ready availability of it is essential in the choosing of a habitat. Water's high heat capacity (the amount of energy needed to increase the temperature of 1gm of water by 1 Degrees Celcius) and high heat of vapourization (amount of energy absorbed by 1gm of liquid to be converted to the gaseous form) prevents from plants and animals from overheating and dying.
Carbohydrates, Lipids, and Proteins.
[edit | edit source]Define organic
- Organic compounds are defined as compounds containing carbon that are found in living organisms.
- Compounds are considered inorganic when they contain carbon but are widely found in the environment (carbon dioxide and hydrogen).
Draw the basic structure of a generalized amino acid
-
Generalized amino acid.
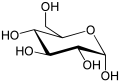
Draw the ring structure of glucose and ribose
-
Glucose. -
Ribose.
Draw the structure of glycerol and a generalized fatty acid [1]click link for picture
Outline the role of condensation and hydrolysis in the relationship between monosaccharides, disaccharides, and polysaccharides; fatty acids, glycerol and glycerides; amino acids, dipeptides, and polypeptides.
- In a condensation reaction, two molecules work together and form one big molecule along with water, because water is released during this reaction. So, two amino acids could join together and form a dipeptide and this would be a condensation reaction. Same applies for monosaccharides becoming disaccharides, you get the drift.
- Now in a hydrolysis reaction, water molecules are used up to make a large molecule into a small molecule. Think about it- "hydro" means water and "lysis" means splitting. So, water is used up to split a disaccharide into a monosaccharide.
Draw the structure of a generalized dipeptide showing peptide linkage http://homepages.ius.edu/GKIRCHNE/peptide.jpg
List three examples for each of monosaccharides, disaccharides, and polysaccharides
- Monosaccharide: Glucose, Fructose and ribose
- Disaccharide: Maltose (Glucose + Glucose) and Sucrose (Glucose + Fructose), and lactose.
- Polysaccharide: Starch (made of glucose subunits) and Glycogen (made of glucose subunits, but linked differently from starch). (Plants use mostly starch, humans use mostly glycogen) and cellulose.
State one function of glucose, lactose and glycogen in animals, and of fructose, sucrose and cellulose in plants.
- Animals
- Glucose: metabolized for energy.
- Lactose: provides energy to mammalian young.
- Glycogen: longer term energy in muscles and liver.
- Plants
- Fructose: sweetens fruit to disperse seeds
- Sucrose: energy storage
- Cellulose: cell wall structure
- Animals
State three functions of lipids
- Energy storage: Fat in humans. Oil in plants.
- Heat insulation: A layer of fat under the skin reduces heat loss.
- Buoyancy: Lipids are less dense than water.
Discuss the use of carbohydrates and lipids in energy storage.
- Lipids and carbohydrates are excellent for storing energy in living organisms. Carbohydrates are usually used to store energy in the short-term while lipids are used for the long-run.
- Advantages of Lipids
- Contain more energy per gram. Therefore lighter to store.
- Lipids are insoluble in water; do not interfere with osmosis.
- Advantages of Carbohydrates
- More easily digested, so energy is released more easily from them.
- Carbohydrates are soluble in water, so easier to transport.
Explain the four levels of protein structure
Primary structure
- A linear sequence of amino acids joined by peptide linkages. There are about 20 different amino acids.
Secondary structure
- α -Helix is maintained by hydrogen bonds. The helix makes up keratin (skin, nails, hair).
- β -Pleated Sheet: flat zig-zag amino acid chain. The sheets make up fibroin (silk.
- Both structures are fibrous and form structural proteins
Tertiary structure
- Folded polypeptide chains into specific shapes. This means that tertiary proteins are globular (hormones, enzymes, membrane proteins).
Quaternary structure
- Two or more polypeptide chains joined (e.g. haemoglobin).
Outline the difference between fibrous and globular proteins, with references to two examples of each protein type
- Fibrous proteins (such as keratin, collagen)
- Chain extended
- Insoluble
- Resistance to pH/temperature changes
- Structural material
- Globular proteins (such as haemoglobin, amylase)
- Chain folded
- Soluble/colloidal
- Susceptible to pH/temperature changes
- Compact, rounded molecules
Enzymes
[edit | edit source]Define Enzyme and Active Site
- Enzyme: Globular proteins used to catalyze chemical reactions.
- Active site: The binding site on the surface of an enzyme where catalysis occurs.
Explain enzyme-substrate specificity
- The active site for an enzyme is very specific in shape, with very precise chemical properties. Active sites match the shape of their substrates. Other molecules do not fit or do not have the same chemical properties. Therefore the enzyme is substrate specific. This enzyme is a lock, and the substrate is the key which can open it.
Explain the effects of temperature, pH and substrate concentration on enzyme activity
- Temperature, pH and substrate concentration all affect the rate at which enzymes catalyse chemical reactions.
Substrate concentration: At low s.c. the enzyme activity is proportional to the substrate concentration, because of random collision between substrate and enzyme. Thus the more substrate the higher the rate. However at a high substrate concentration, at some point all active sites are occupied so raising the substrate concentration has no effect.
Temperature: Enzyme activity increases as temperature increases, often doubling with each 10°C. This is because collision between the substrate and active site happen more frequently at higher temperatures, due to fast molecular movement. However, at high temperatures enzymes are denatured and stop working. This is because heat causes vibrations inside the enzyme, which break bonds needed to maintain the structure.
pH: There is an optimum at which enzyme activity is fastest ( mostly pH 7), and as pH increases or decreases from its optimum, enzyme activity is reduced. (acids and alkali denature enzymes)
Define Denaturation
- Denaturation: A structural change in a protein that results in a loss of its biological properties. This can be caused by pH or by temperature.
Explain the use of lactase in the production of lactose free milk
Lactose- The sugar present in milk. Lactase is obtained from Kluveromyces and lactis, a type of yeast that grows naturally in milk.
Lactose can be converted to glucose and galactose using the enzyme lactase (di-saccarharide).
Biotechnology companies culture the yeast and extract the enzyme in order to produce lactose free milk.
- Advantages: Pectinase makes juice more fluid and easy to separate from pulp.
- Fructose is widely used in food manufacturing because it is much sweeter than glucose. It is made from starch, usually found in maize. Amylase is needed to break down the starch into glucose.
- 'Source of Enzyme: Amylase is obtained from fungi.
- Use: Used to break Starch into glucose, which is then converted into fructose using the enzyme glucose isomerase.
DNA Structure
[edit | edit source]Outline DNA nucleotide structure in terms of sugar (deoxyribose), base, and phosphate.
- A DNA is composed of a deoxyribose, a phosphate group and a nitrogen base (Adenine, Cytosine, Thymine, and Guanine). The phosphate group is covalently bonded to the carbon of the deoxyribose and then the nitrogenous base is attached to the deoxyribose.
State the four names of the bases of DNA
- Adenine, Cytosine, Guanine, and Thymine.
Outline how the DNA nucleotides are linked together by covalent bonds into a single strand.
- Two DNA nucleotides can be linked together by a covalent bond between the sugar of one nucleotide and the phosphate of another. More nucleotides can be added to form a single strand.
Explain how the DNA double helix is formed using complementary base pairing and hydrogen bonds.
- DNA molecules consist of two strands of nucleotides which are then wound together to form a double helix. These are formed between the bases of two strands. However, it is formed by complementary base pairing because Adenine only forms hydrogen bonds with Thymine and Cytosine only forms hydrogen bonds with Guanine.
Draw a simple diagram of the molecular structure of DNA.
URL of a suitable picture: http://www.cem.msu.edu/~reusch/VirtTxtJml/Images3/dblhelx1.gif
DNA Replication
[edit | edit source]State that DNA replication is semi-conservative.
- DNA replication copies DNA to produce new molecules with the same base sequence. It is semi-conservative because each new molecule formed by replication uses one new strand and one old strand which is conserved from the parent DNA molecule.
Explain DNA replication in terms of unwinding the double helix and separation of the strands by helicase, followed by the formation of the new complementary strands by DNA polymerase.
- Stage 1: The DNA double helix is unwound and separated into strands by helicase breaking the hydrogen bonds holding the strands together and creating a replication bubble.
- Stage 2: The single strands act as blueprints for new strands. Free nucleotides are present in large numbers. The enzyme primase places primers on the new strand, allowing DNA Polymerase III to add to the strand in a 5-carbon to 3-carbon direction. Several small strands are made within the replication bubble formed by helicase that are known as Okazaki fragments. DNA Polymerase III is then removed, and DNA polymerase I replaces primers with corresponding base pairs. The enzyme ligase then binds these new base pairs to the rest of the strand. The bases of these nucleotides form hydrogen bonds with the bases of the parent strand. The nucleotides are connected to form a new strand.
- Stage 3: The daughter DNA molecules each rewind into a double helix.
The two daughter DNA molecules are identical in base sequence to each other and to the parent molecule, because of complementary base pairing (Adenine pairs with Thymine and Cytosine with Guanine). Each of the new strand is complementary to the template on which it was made and identical to the other template.
Explain the significance of complementary base pairing in the conservation of the base sequence of DNA.
- Because the nitrogenous bases that compose DNA can only pair with complementary bases, any two linked strands of DNA are complementary. This ensures that the old base sequence is conserved.
Transcription and Translation
[edit | edit source]Compare the structure of RNA and DNA
- 1. The number of strands.
- DNA has two strands forming a double helix.
- RNA has one strand.
- 2. The type of sugar.
- DNA has deoxyribose.
- RNA has a Ribose.
- 3. The Nucleotides.
- DNA has A,C,G,T
- RNA has A,C,G,U, (Uracil replaces Thymine)
Outline DNA transcription in terms of the formation of the RNA strand complementary to the DNA strand by RNA polymerase.
- Transcription: The copying of the base sequence of a gene by making an RNA molecule.
- The DNA double helix uncoils and the two strands separate.
- RNA Polymerase attaches to promoter regions of the DNA strand
- RNA polymerase binds free RNA nucleotides to create mRNA that are a copied template of the corresponding DNA strand.
- The mRNA separates from the DNA.
- The DNA strands reforms into a double helix.
Describe the genetic code in terms of codons composed of triplets and bases. The genetic code is a triplet code- three bases code for one amino acid. A group of three bases is called a codon.
Explain the process of translation, leading to polypeptide formation
- Before the mRNA strand is translated, it must be processed. A cap and tail are added to the mRNA strand to allow the mRNA strand out of the nucleus.
- There are intron and exon segments of the strand. The introns are removed by the introduction of a splicesome. The splicesome pushes out the introns and binds the exons together.
- The mRNA then passes through the nuclear pore and enters the cytoplasm.
- The two subunits of a ribosome (60s and 40s) lock onto the mRNA at the ribosome-binding site.
- Transfer RNA (tRNA) are clover-leafed shaped molecules that have a specific amino acid attached.
- Each tRNA also has three bases called the anticodon that binds to the mRNA. The anticodon determines which amino acid the tRNA carries.
- The tRNA can only bind to the mRNA in the presence of a ribosome. In each ribosome there are two tRNA binding sites (Aminoacyl tRNA binding site or A site and Peptidinal tRNA binding site or P site) and a tRNA exit site (E site).
- The mRNA is divided into groups of three bases each called a codon. The anticodon on the tRNA binds to the complementary codon on the mRNA. In so doing it brings the amino acid into position.
- The mRNA is fed between the small and large subunits of the ribosome. The first tRNA molecule with the attached start amino acid, methanine, attaches at the A site and is transferred to the P site. The next tRNA binds to the mRNA and contains an amino acid.
- When two tRNA molecules are bound to the mRNA at the ribosome a ribosomal enzyme forms a peptide bond between the two amino acids.
- The first tRNA becomes detached from its amino acid and moves off from the ribosome after it slides into the exit site.
- The ribosome moves a distance of one codon and a new tRNA binds bringing in another amino acid to be joined to the polypeptide chain.
- The first codon translated on any mRNA is always AUG. This is called the initiation codon and it sets the reading frame of the mRNA.
- There are also three codons that signal the end of the mRNA and stop translation. They are the stop codons UAG, UAA and UGA.
- Several ribosomes may all be translating the same mRNA at the same time. This is called a polysome.
Define the terms degenerate, and universal as they relate to the genetic code
- Degenerate: Having more than one base triplet to code for one amino acid.
- Universal: Found in all living organisms.
Discuss the relationship between one gene and one polypeptide
- Polypeptides are long chains of amino acids.
- Amino acids must be linked up in a precise sequence to make a polypeptide.
- Genes store the information needed to make a polypeptide in a coded form.
- The sequence of bases in a gene codes for the sequence of amino acids in a polypeptide.
- The information in the gene is decoded during the making of the polypeptide.
- This process is conducted in two stages known as transcription and translation.
Cell Respiration
[edit | edit source]Define Cell-Respiration
- Cell Respiration: controlled release of energy in the form of ATP from organic compounds in cells.
In cell respiration, glucose in the cytoplasm is broken down into pyruvate with a small yield of ATP
- Glucose is an organic compound that is sometimes used in cell respiration. Chemical reactions break Glucose into a simpler compound called pyruvate. A small amount of ATP (2 ATP) is produced by using energy released from glucose.
In anaerobic cell respiration pyruvate is converted into lactate or ethanol dioxide in the cytoplasm with no further yield of ATP
- Lactic fermentation: In the process of glycolysis glucose is transferred into two pyruvate. Since no oxygen is available, the Krebs cycle cannot run which therefore also stops the process of the electron transport chain. Too much pyruvate is built up in the cytoplasm and we need to get rid of it. Therefore, it is transferred into the waste product - lactate - that can be removed from the cell. No ATP is produced.
- Alcohol fermentation: since there is no oxygen, pyruvate is transferred into ethanol/alcohol and carbon dioxide is created as a bi-product. This is how one-celled organisms overcome oxygen deficiency.
In aerobic respiration pyruvate is broken down in the mitochondrion into carbon dioxide and water with a large yield of ATP If oxygen is available, the pyruvate is absorbed by mitochondrion. The pyruvate is then broken down into carbon dioxide and water. A large amount of ATP is produced.
- Note: How to remember the difference! Aerobic sounds healthy- so something good comes out of it! Anaerobic sounds like unaerobic and that sounds unhealthy! Easy to remember, right? Also, think of doing aerobics, which is healthy as well and makes you breathe a lot!
Photosynthesis
[edit | edit source]State that photosynthesis involves the conversion of light energy into chemical energy.
- Photosynthesis involves energy conversion. Light energy, usually sunlight, is converted into chemical energy.
State that white light from the sun is composed of a range of wavelengths.
- Sunlight is called white light, but it is actually made up of a range of wavelengths including red, blue, and green.
State that chlorophyll is the main photosynthetic pigment
- The structure of chlorophyll allows it to absorb some colors of wavelength better than others.
Outline the differences in absorption of red, blue and green light by chlorophyll
- Red and blue light are absorbed more than green. The green light that cannot be absorbed is reflected giving plants (and the pigment chlorophyll) their green color.
State that light energy is used to split water molecules (photolysis) to give oxygen and hydrogen and to produce ATP
- Some of the energy absorbed by chlorophyll is used to produce ATP
- Some of the energy absorbed by chlorophyll is used to split water molecules. This is called photolysis of water
- Photolysis of water results in the formation of oxygen and hydrogen. The oxygen is released as a waste product
State that ATP and hydrogen (derived from photolysis of water) are used to fix carbon dioxide to make organic molecules.
- Carbon dioxide is absorbed for use in photosynthesis
- The carbon from it is used to make a wide range of organic substances
- The conversion of carbon in gas to carbon in solid compounds is called carbon fixation
- Carbon fixation involves the use of hydrogen from photolysis and energy from ATP.
Explain that the rate of photosynthesis can be measured directly by the production of oxygen or the uptake of carbon dioxide, or indirectly by the increase of biomass
- Uptake of carbon dioxide: Since carbon dioxide is important in the light-independent reactions of photosynthesis, its consumption by plants can be measured as a means to determine the rate of ATP and electron carriers used for carbon fixation.
- Production of oxygen: Aquatic plants release bubbles of oxygen when they carry photosynthesis. e.g. these bubbles are collected; their volume can be measured.
- Increase in Biomass: If batches of plants are harvested at a series of times and their biomass determined, then the rate of photosynthesis can be determined by an increase in biomass.
Outline the effects of temperature, light intensity, and carbon dioxide concentration on the rate of photosynthesis:
- Light - At low medium light intensities the rate is directly proportional to light intensity. At high light intensities the rate reaches a plateau.
- Carbon Dioxide - No photosynthesis at very low CO2 concentrations. At low to fairly high CO2 concentrations the rate is positively correlated with CO2 concentration. At very high CO2 concentrations the rate reaches a plateau.
- Temperature - As temperature increases the rate increases more and more steeply. If the temperature increases with 10¤C it roughly doubles the rate. When it reaches it maximum point it is said to be its Optimum Temperature which is around 40¤C. Above the optimum temperature the rate slows down rapidly and then stops. This happens because the excessive heat destroys the enzymes which are responsible for catalyzing chemical reactions.