Applied Science BTEC Nationals/Practical Chemical Analysis
Analytical chemistry is the science that seeks ever-improved means of measuring the chemical composition of materials. Chemical composition is the entire picture (composition) of the material at the chemical scale and includes geometric features such as molecular morphologies and distributions of species within a sample as well as single-dimensional features such as percent composition and species identity. The analytical results enabled by analytical chemistry have played critical roles in science from the understanding of basic science to a variety of practical applications, such as biomedical applications, environmental monitoring, quality control of industrial manufacturing and w:forensic science.
Overview
[edit | edit source]Analytical chemistry is a sub discipline of w:chemistry that has the broad mission of understanding the chemical composition of all matter and developing the tools to elucidate such compositions. This differs from other sub disciplines of chemistry in that it is not intended to understand the physical basis for the observed chemistry as with w:physical chemistry and it is not intended to control or direct chemistry as is often the case in w:organic chemistry and it is not necessarily intended to provide engineering tactics as are often used in w:materials science. Analytical chemistry generally does not attempt to use chemistry or understand its basis; however, these are common outgrowths of analytical chemistry research. Analytical chemistry has significant overlap with other branches of chemistry, especially those that are focused on a certain broad class of chemicals, such as organic chemistry, w:inorganic chemistry or w:biochemistry, as opposed to a particular way of understanding chemistry, such as w:theoretical chemistry. For example the field of bioanalytical chemistry is a growing area of analytical chemistry that addresses all analytical questions in biochemistry, (the chemistry of life). Analytical chemistry and experimental physical chemistry, however, have a unique relationship in that they are very unrelated in their mission but often share the most in common in the tools used in experiments.
Analytical chemistry is particularly concerned with the questions of "what chemicals are present, what are their characteristics and in what quantities are they present?" These questions are often involved in questions that are more dynamic such as what w:chemical reaction an w:enzyme catalyses or how fast it does it, or even more dynamic such as what is the w:transition state of the reaction. Although analytical chemistry addresses these types of questions it stops after they are answered. The logical next steps of understanding what it means, how it fits into a larger system, how can this result be generalised into theory or how it can be used are not analytical chemistry. Since analytical chemistry is based on firm experimental evidence and limits itself to some fairly simple questions to the general public it is most closely associated with hard numbers such 'as how much lead is in drinking water'.
Modern analytical chemistry
[edit | edit source]Modern analytical chemistry is dominated by instrumental analysis. There are so many different types of instruments today that it can seem like a confusing array of acronyms rather than a unified field of study. Many analytical chemists focus on a single type of instrument. Academics tend to either focus on new applications and discoveries or on new methods of analysis. The discovery of a chemical present in blood that increases the risk of cancer would be a discovery that an analytical chemist might be involved in. An effort to develop a new method might involve the use of a w:tunable laser to increase the specificity and sensitivity of a spectrometric method. Many methods, once developed, are kept purposely static so that data can be compared over long periods of time. This is particularly true in industrial quality assurance (QA), forensic and environmental applications. Analytical chemistry plays an increasingly important role in the pharmaceutical industry where, aside from QA, it is used in discovery of new drug candidates and in clinical applications where understanding the interactions between the drug and the patient are critical.
Types
[edit | edit source]Traditionally, analytical chemistry has been split into two main types, qualitative and quantitative:
Qualitative
[edit | edit source]- w:Qualitative inorganic analysis seeks to establish the presence of a given element or w:inorganic compound in a sample.
- Qualitative organic analysis seeks to establish the presence of a given w:functional group or w:organic compound in a sample.
Quantitative
[edit | edit source]- Quantitative analysis seeks to establish the amount of a given element or compound in a sample.
Approaches
[edit | edit source]Most modern analytical chemistry is categorised by two different approaches such as analytical targets or analytical methods. w:Analytical Chemistry (journal) reviews two different approaches alternatively in the issue 12 of each year.
By Analytical Targets
[edit | edit source]- Bioanalytical chemistry
- Material analysis
- Chemical analysis
- Environmental analysis
- Forensics
By Analytical Methods
[edit | edit source]- w:Spectroscopy
- w:Mass Spectrometry
- w:Spectrophotometry and w:Colorimetry
- w:Chromatography and w:Electrophoresis
- w:Crystallography
- w:Microscopy
- w:Electrochemistry
Traditional analytical techniques
[edit | edit source]Although modern analytical chemistry is dominated by sophisticated instrumentation, the roots of analytical chemistry and some of the principles used in modern instruments are from traditional techniques many of which are still used today. These techniques also tend to form the backbone of most undergraduate analytical chemistry educational labs. Examples include:
Titration
[edit | edit source]Titration involves the addition of a reactant to a solution being analysed until some equivalence point is reached. Often the amount of material in the solution being analyzed may be determined. Most familiar to those who have taken college chemistry is the acid-base titration involving a colour-changing indicator. There are many other types of titrations, for example potentiometric titrations.
Gravimetry
[edit | edit source]Gravimetric analysis involves determining the amount of material present by weighing the sample before and/or after some transformation. A common example used in undergraduate education is the determination of the amount of water in a hydrate by heating the sample to remove the water such that the difference in weight is due to the water lost.
Inorganic qualitative analysis
[edit | edit source]Inorganic qualitative analysis generally refers to a systematic scheme to confirm the presence of certain, usually aqueous, ions or elements by performing a series of reactions that eliminate ranges of possibilities and then confirms suspected ions with a confirming test. Sometimes small carbon containing ions are included in such schemes. With modern instrumentation these tests are rarely used but can be useful for educational purposes and in field work or other situations where access to state-of-the-art instruments are not available or expedient.
Instrumental Analysis
[edit | edit source]Spectroscopy
[edit | edit source]Spectroscopy measures the interaction of the molecules with electromagnetic radiation. Spectroscopy consists of many different applications such as w:atomic absorption spectroscopy, atomic emission spectroscopy, w:ultraviolet-visible spectroscopy, w:infrared spectroscopy, w:Raman spectroscopy, w:nuclear magnetic resonance spectroscopy, w:photoemission spectroscopy, w:Mössbauer spectroscopy and so on.
UV-Vis Spectroscopy
[edit | edit source]Coloured chemicals absorb electromagnetic waves in the visible part of the spectrum. The absorbed energy
causes changes in the energy of the molecules’ electrons. The electrons change from a ‘ground state’ to an ‘excited state’.
Most transitions are not caused by visible light. Many absorb ultra-violet radiation. Chemicals which absorb UV radiation are colourless (unless they fluoresce). The energy changes when molecules of a coloured compound and of a colourless compound are illustrated below:
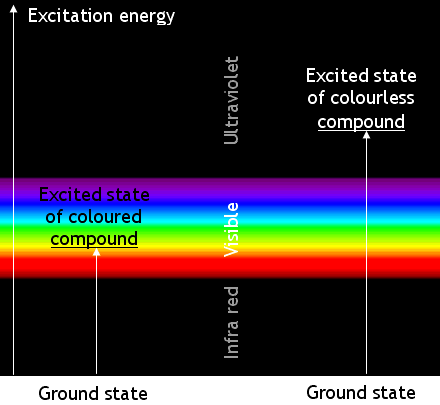
Remember that the apparent colour is caused by absorbing photons of a complementary colour. A blue compound is blue because it absorbs yellow light.

Chromophores
[edit | edit source]Chemical structures which have excited states corresponding to visible light are calledchromophores. There are two main types:
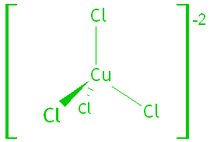
1. Transition Metal Complexes.
Transition metals form complex ions – the metal binds to small molecules or anions called ligands. The ligands allow the electrons of the metal ion to enter an excited state if the electrons absorb a photon of visible light.
e.g. tetrachlorocuprate (II) and hexaaquacopper (II) ions:
The partially-occupied d-orbitals of transition metal compounds are important in giving colour to transition metal complexes. See diagram (which could represent V+2, Cr+3, Mn+4, etc.):

①. In an uncomplexed ion, all the d-orbitals have the same energy.
②. When ligands surround the ion, the negative charges of the ligands make the d-orbitals less stable (higher energy).
③. Critically, the ligands will come closer to some d-orbitals than to others. Typically, two or three of the orbitals will be destabilised more than the remainder.
An electron in one of the lower d-orbitals can acquire the energy to be excited into a higher d-orbital:

This mechanism allows transition metal complexes to absorb photons of visible light.
2. Conjugated/Delocalised Electron Systems.
When single and double bonds alternate, the electrons in the double bonds can enter an excited state if they absorb a photon of visible light. e.g. β-carotene (above) has ten conjugated C=C bonds:
The diagram above shows the excitation energies of conjugated aldehydes. n is the number of C=C double bonds which are conjugated. The simplest (n=1) is CH3-CH=CH-CH=O. Note how the excitation energy is lower with higher numbers of conjugated bonds.
| n | Wavelength (nm) | Energy (kJ mol−1) |
| 1 | 220 | 544 |
| 2 | 270 | 443 |
| 3 | 312 | 384 |
| 4 | 343 | 349 |
| 5 | 370 | 324 |
| 6 | 393 | 305 |
| 7 | 415 | 289 |
Chromophores of dye molecules often contain unsaturated groups such as >C=O and -N=N-, which are part of a conjugated bonding system, usually involving aromatic rings. Chrysoidine, a basic dye, is shown below:

Note how the –N=N- group is just the centre of a conjugated system which extends across all twelve carbon atoms and includes seven double bonds. All azo dyes contain the -N=N- arrangement.
Auxochromes: Attached to the chromophore are two -NH2 groups which interact with the chromophore to modify the orange colour. A group of atoms attached to a chromophore which modifies the ability of that chromophore to absorb light is called an auxochrome. They can modify or enhance the colour of the dye. Examples: -OH , - NH2, aldehydes.
Added functional groups can also:
- alter the solubility of the dye in water or other solvents.
- bind the dye molecules to cloth, paper or other substrates.

References
[edit | edit source]Notes on colour chemistry by elecuter.
See also
[edit | edit source]Notes on colour chemistry at Bristol University.
Mass Spectrometry
[edit | edit source]w:Mass Spectrometry Mass spectrometry measures mass-to-charge ratio of molecules using electric and w:magnetic fields. There are several ionization methods: electron impact, chemical ionisation, electrospray, matrix assisted laser desorption ionisation, and others. Also, mass spectrometry is categorised by approaches of mass analyers: w:magnetic-sector, w:quadrupole mass analyser, w:quadrupole ion trap, w:Time-of-flight, w:Fourier transform ion cyclotron resonance, and so on.
Crystallography
[edit | edit source]w:Crystallography
Crystallography is a technique that characterises the chemical structure of materials at the atomic level by analysing the w:diffraction patterns of (usually) w:x-rays that have been deflected by atoms in the material. From the raw data the relative placement of atoms in space may be determined.
Electrochemical Analysis
[edit | edit source]w:Electrochemistry Electrochemistry measures the interaction of the material with an w:electric field.
Thermal Analysis
[edit | edit source]w:Calorimetry, w:thermogravimetric analysis Calorimetry and thermogravimetric analysis measure the interaction of a material and w:heat.
Separation
[edit | edit source]Separation processes are used to decrease the complexity of material mixtures. w:Chromatography and w:electrophoresis are representative of this field.
Hybrid Techniques
[edit | edit source]Combinations of the above techniques produce "hybrid" or "hyphenated" techniques. Several examples are in popular use today and new hybrid techniques are under development. For example, w:Gas chromatography-mass spectrometry, LC-MS, GC-IR, LC-NMR, CE-MS, and so on.
Hyphenated separation techniques refers to a combination of two (or more) techniques to detect and separate chemicals from solutions. Most often the other technique is some form of w:chromatography. Hyphenated techniques are widely used in w:chemistry and w:biochemistry.
A slash is sometimes used instead of w:hyphen, especially if the name of one of the methods contains a hyphen itself.
List of hyphenated techniques:
Microscopy
[edit | edit source]The visualization of single molecules, single cells, biological tissues and nano- micro materials is very important and attractive approach in analytical science. Also, hybridization with other traditional analytical tools is revolutionizing analytical science. Microscopy can be categorized into three different fields: optical microscopy, electron microscopy, and scanning probe microscopy. Recently, this field is rapidly progressing because of the rapid development of computer and camera industries.
Lab-on-a-chip
[edit | edit source]Miniaturized analytical instrumentation, which is also called as microfluidics or micro total analysis system (μTAS). The beauty of lab-on-a-chip system is that a whole device can be visualized under a microscope.
Methods and data analysis
[edit | edit source]Standard Curve
[edit | edit source]A standard method for analysis of concentration involves the creation of a w:calibration curve. This allows for determination of the amount of a chemical in a material by comparing the results of unknown sample to those of a series known standards.If the concentration of element or compound in a sample is too high for the detection range of the technique, it can simply be diluted in a pure solvent. If the amount in the sample is below an instrument's range of measurement, the method of addition can be used. In this method a known quantity of the element or compound under study is added, and the difference between the concentration added, and the concentration observed is the amount actually in the sample.
Internal Standards
[edit | edit source]Sometimes an w:internal standard is added at a known concentration directly to an analytical sample to aid in quantitation. The amount of analyte present is then determined relative to the internal standard as a calibrant.
Trends
[edit | edit source]Analytical chemistry research is largely driven by performance (sensitivity, selectivity, robustness, w:linear range, accuracy, precision, and speed), and cost (purchase, operation, training, time, and space).
A lot of effort is put in shrinking the analysis techniques to w:chip size. Although there are few examples of such systems competitive with traditional analysis techniques, potential advantages include size/portability, speed, and cost. (micro w:Total Analysis System (µTAS) or w:Lab-on-a-chip). w:Microscale chemistry reduces the amounts of chemicals used.
Much effort is also put into analysing biological systems. Examples of rapidly expanding fields in this area are:
- w:Genomics - w:DNA sequencing and its related research. w:Genetic fingerprinting and w:DNA microarray are very popular tools and research fields.
- w:Proteomics - the analysis of protein concentrations and modifications, especially in response to various stressors, at various developmental stages, or in various parts of the body.
- w:Metabolomics - similar to proteomics, but dealing with metabolites.
- w:Transcriptomics- mRNA and its associated field
- w:Lipidomics - lipids and its associated field
- Peptidomics - peptides and its associated field
- Metalomics - similar to proteomics and metabolomics, but dealing with metal concentrations and especially with their binding to proteins and other molecules.
Analytical chemistry has played critical roles in the understanding of basic science to a variety of practical applications, such as biomedical applications, environmental monitoring, quality control of industrial manufacturing, forensic science and so on.
The recent developments of computer automation and information technologies have innervated analytical chemistry to initiate a number of new biological fields. For example, automated DNA sequencing machines were the basis to complete human genome projects leading to the birth of w:genomics. Protein identification and peptide sequencing by mass spectrometry opened a new field of w:proteomics. Furthermore, a number of ~omics based on analytical chemistry have become important areas in modern biology.
Also, analytical chemistry has been an indispensable area in the development of w:nanotechnology. Surface characterization instruments, w:electron microscopes and scanning probe microscopes enables scientists to visualize atomic structures with chemical characterizations.
Analytical chemistry is pursuing the development of practical applications and commercial instruments rather than elucidating scientific fundamentals. This may be an arguable difference from overlapping science areas such as physical chemistry and biophysics, although there isn't any distinct boundaries among disciplines in contemporary science and technology. However, this aspect may attract many engineers' interest; thus, it is not difficult to see papers from engineering departments in analytical chemistry journals.
Among active contemporary analytical chemistry research fields, micro w:total analysis system is considered as a great promise of revolutionary technology. In this approach, integrated and miniaturised analytical systems are being developed to control and analyze single cells and single molecules. This cutting-edge technology has a promising potential of leading a new revolution in science as integrated circuits did in computer developments.
See also
[edit | edit source]- w:List of chemical analysis methods
- w:List of materials analysis methods
- Important publications in analytical chemistry
- American Chemical Society: Division of Analytical Chemistry
- Royal Society of Chemistry: Analytical Gateway
References
[edit | edit source]- ↑ Streitwieser, A & Heathcock, CH (1985) Introduction to organic chemistry (3rd ed) p 628, Macmillan, New York
Further reading
[edit | edit source]- Journal of AOAC International ISSN: 1060-3271, AOAC International
Sources
[edit | edit source]Adapted from w:Analytical chemistry
Experiments
[edit | edit source]Measuring sulphate concentration.
Measuring copper content of brass.
Measuring manganese content of steel.
Measuring calcium concentration by titration.
Measuring iron concentration by colorimetry.
Standard solutions
[edit | edit source]Be able to prepare and standardise solutions of specified concentrations
Molarity: calculations involving molar quantities; calculation of concentration including use of dilution factors necessary to produce a range of standard solutions from a given stock solution of known concentration
Standard solutions: preparation of solutions of fixed concentration; appropriate titrations to determine concentration or standardise given solutions; dilution of stock solutions to give a series of related standard solutions
Spectroscopy
[edit | edit source]Understand the design and operating principles of selected spectroscopic instruments and be able to use spectroscopic methods to analyse chemical substances
Spectroscopic instruments: e.g. ultraviolet/visible spectroscopy, infrared spectroscopy, 1H NMR spectroscopy, atomic spectroscopy, mass spectrometry; block diagrams showing key components; basic principles of operation e.g. energy sources, optics, magnets, detectors
Spectroscopic techniques: e.g. infrared spectroscopy, absorption bands and correlation charts, identification of organic functional groups, origin and uses of the fingerprint region; ultraviolet/visible spectroscopy, Beer-Lambert law, measurement of absorbance, construction of calibration curves, measurement of concentration, determination of molar absorption coefficients; atomic spectroscopy, applications of absorption and emission spectroscopy, criteria for method selection, use in quantitative analysis, calibration curves and internal standards; 1H NMR spectroscopy, conditions for NMR activity, examples of other NMR active nuclei in addition to H, TMS as internal standard, correlation charts, integration traces, spin-spin splitting, identification of simple organic compounds from 1H NMR spectra; mass spectrometry, measurement of relative molecular mass, simple fragmentation patterns
Chromatography
[edit | edit source]Understand the principles of chromatographic separation of components and be able to use chromatographic methods to separate and analyse chemical substances
Chromatographic principles: stationary and mobile phases; adsorption (liquidsolid) chromatography; liquid-liquid chromatography; gas-liquid chromatography (GLC); ion exchange chromatography; Rf values; visualisation/detection of fractions; quantitative and qualitative uses; basic instrumentation (where appropriate)
Chromatographic methods: e.g. practical applications of chromatographic separations e.g. paper, column, thin layer, GLC, high performance liquid chromatography (HPLC), ion exchange, molecular exclusion (gel permeation) chromatography
Chemical substances: simple mixtures e.g. glucose-maltose mixture, seven food dye mixture dissolved in water (Erythrosin, Brilliant Black BN, Fast Red E, Naphthol Red S, Yellow Orange S, Ponceau 4R and Tartrazine)
Paper chromatography
[edit | edit source]'Paper chromatography is an analytical technique for separating and identifying compounds that are or can be coloured, especially pigments. This method has been largely replaced by thin layer chromatography, however it is still a powerful teaching tool. Two-way paper chromatography, also called two-dimensional chromatography, involves using two solvents and rotating the paper 90° in between. This is useful for separating complex mixtures of similar compounds, for example, amino acids.
Technique
[edit | edit source]
A small spot of solution containing the sample is applied to a strip of chromatography paper about 1 cm from the base. This sample is adsorbed onto the paper and may form interactions with it. Any substance that reacts or bonds with the paper cannot be measured using this technique. The paper is then dipped in to a suitable solvent such as ethanol or water, and placed in a sealed container.
The solvent moves up the paper by capillary action, which occurs as a result of the attraction of the solvent molecules to the paper and to one another. As the solvent rises through the paper it meets and dissolves the sample mixture, which will then travel up the paper with the solvent. Different compounds in the sample mixture travel at different rates due to differences in solubility in the solvent, and due to differences in their attraction to the fibres in the paper. Paper chromatography takes anywhere from several minutes to several hours.
In some cases, paper chromatography does not separate pigments completely; this occurs when two substances appear to have the same Rf values in a particular solvent. In these cases, two-way chromatography is used to separate the multiple-pigment spots. The chromatogram is turned by ninety degrees, and placed in a different solvent in the same way as before; some spots separate into multiple spots, showing the presence of more than one pigment. As before the Rf value is calculated, and the two pigments identified.
Thin-layer chromatography
[edit | edit source]
Thin layer chromatography (TLC) is a widely-used chromatography technique used to separate chemical compounds. It involves a stationary phase consisting of a thin layer of adsorbent material, usually silica gel, alumina, or cellulose immobilised onto a flat, inert carrier sheet. A liquid phase consisting of the solution to be separated dissolved in a solvent is drawn through the plate via capillary action, separating the experimental solution. It can be used to determine the pigments a plant contains, to detect pesticides or insecticides in food, in forensics to analyse the dye composition of fibres, or to identify compounds present in a given substance, among other uses.
Plate preparation
[edit | edit source]TLC plates are made by mixing the adsorbent, such as silica gel, with a small amount of inert binder like calcium sulphate (gypsum) and water. This mixture is spread as a thick slurry on an inert carrier sheet, usually glass, thick aluminium foil, or plastic, and the resultant plate is dried and activated by heating in an oven. The thickness of the adsorbent layer is typically around 0.1–0.25 mm for analytical purposes and around 1–2 mm for preparative TLC.
Technique
[edit | edit source]The process is similar to paper chromatography with the advantage of faster runs, better separations, and the choice between different stationary phases. Because of its simplicity and speed TLC is often used for monitoring chemical reactions and for the qualitative analysis of reaction products.
A small spot of solution containing the sample is applied to a plate, about one centimeter from the base. The plate is then dipped in to a suitable solvent, such as ethanol or water, and placed in a sealed container. The solvent moves up the plate by capillary action and meets the sample mixture, which is dissolved and is carried up the plate by the solvent. Different compounds in the sample mixture travel at different rates due to differences in solubility in the solvent, and due to differences in their attraction to the stationary phase.
Analysis of chromatograms
[edit | edit source]After development, the spots corresponding to different compounds may be located by their colour, by ultraviolet light, ninhydrin or by treatment with iodine vapours. The final chromatogram can be compared with other known mixture chromatograms to identify sample mixes using the Rf value.
As in most other forms of chromatography, paper chromatography uses Rf values to help identify compounds. Rf values are calculated by dividing the distance the pigment travels up the paper by the distance the solvent travels (the solvent front). Because Rf values are standard for a given compound, known Rf values can be used to aid in the identification an unknown substance in an experiment.
As the chemicals being separated may be colourless, several methods exist to visualise the spots:
- Often a small amount of a fluorescent compound, usually Manganese-activated Zinc Silicate, is added to the adsorbent that allows the visualisation of spots under a blacklight(UV254). The adsorbent layer will thus fluoresce light green by itself, but spots of analyte quench this fluorescence.
- Iodine vapours are a general unspecific colour reagent
- Specific colour reagents exist into which the TLC plate is dipped or which are sprayed onto the plate
Once visible, the Rf value of each spot can be determined by dividing the distance travelled by the product by the total distance travelled by the solvent (the solvent front). These values depend on the solvent used, and the type of TLC plate, and are not physical constants.
Sources
[edit | edit source]Operation of industrial and commercial laboratories
[edit | edit source]Understand how an industrial or commercial laboratory operates
Laboratory type: any multifunctional laboratory e.g. hospital clinical chemistry, government public health, industrial quality control laboratory
Processes: range of analytical procedures; data recording and manipulation; data presentation; quality assurance;
See Case studies
Resources
[edit | edit source]Edexcel recommend the following resources except * which have been added to their list.
Textbooks
[edit | edit source]Barker J — Mass Spectrometry (Analytical Chemistry by Open Learning Series) (John Wiley & Sons, 1998) ISBN 0471967629
Dean J R (editor) — Atomic Absorption and Plasma Spectroscopy (Analytical Chemistry by Open Learning Series) (John Wiley & Sons, 2000) ISBN 0471972541
Downard K — Mass Spectrometry: A Foundation Course (Royal Society of Chemistry, 2004) ISBN 0854046097
Fowlis I A — Gas Chromatography (Analytical Chemistry by Open Learning Series) (John Wiley & Sons, 1995) ISBN 0471954683
Hanai T — HPLC: A Practical Guide (RSC Chromatography Monographs) (Royal Society of Chemistry, 1999) ISBN 0854045155
Hill G and Holman J — Chemistry in Context: Laboratory Manual and Student Guide (Chemistry in Context) (Nelson Thornes Ltd, 2001) ISBN 0174483074
Lajunen L H and Peramaki P — Spectrochemical Analysis by Atomic Absorption and Emission, 2nd Edition (Royal Society of Chemistry, 2005) ISBN 0854046240
Levinson R — More Modern Chemical Techniques (Royal Society of Chemistry, 2002) ISBN 0854049290
Lindsay S — High Performance Liquid Chromatography (Analytical Chemistry by Open Learning Series) (John Wiley & Sons, 1992) ISBN 0471931152
Sewell P A and Clarke B — Chromatographic Separations (Analytical Chemistry by Open Learning Series) (John Wiley & Sons, 1987) ISBN 0471913715
Stuart B H — Modern Infrared Spectroscopy (Analytical Chemistry by Open Learning Series) (John Wiley & Sons, 1995) ISBN 0471959170
Thomas M J K — Ultraviolet and Visible Spectroscopy (Analytical Chemistry by Open Learning Series) (John Wiley & Sons, 1996) ISBN 0471967432
CD ROMs
[edit | edit source]Practical Chemistry for Schools & Colleges (Royal Society of Chemistry)
Spectroscopy for Schools & Colleges (Royal Society of Chemistry)
Websites
[edit | edit source]The RSC’s Chemical Science Network



