Organic Chemistry/Amines

Amines are organic compounds which contain and are often actually based on one or more atoms of nitrogen. Structurally amines resemble ammonia in that the nitrogen can bond up to three hydrogens, but amines also have additional properties based on their carbon connectivity. In an amine, one or more of the hydrogen atoms from ammonia are replaced by organic substituents like alkyl (alkane chain) and aryl (aromatic ring) groups.
Another type of organic molecule contains nitrogen without being, strictly speaking, an amine: carboxylic acid derivatives containing a trivalent (three-bond) ammonia in ground state are actually amides instead of amines. Amides and amines have different structures and properties, so the distinction is actually very important. Organic-nitrogen compounds containing metals are also called amides, so if you see a molecule that has a nitrogen and either a carbonyl group or a metal next to that nitrogen, then you know that molecule should be an amide instead of an amine.
Preparation
[edit | edit source]The following laboratory methods can be considered to be in common use for purpose of the preparation of amine compounds:
From Oximes
[edit | edit source]- Aldoximes or ketoximes are reduced by sodium and ethanol to corresponding primary amines
H
|
R-C=N - OH + H ----> Na / Ethanol R-CH2-NH2 + H2O
The Gabriel Phthalimide synthesis
[edit | edit source]
- via azides by the Staudinger reduction.
- Allylic amines can be prepared from amines in the Aza-Baylis-Hillman reaction.
used for preparation of primary amines. Aromatic primary amines can't be prepared by this method because aryl halides do not undergo nueleophilic substitution with potassium phthalimide.
Hofmann degradation of amides
[edit | edit source]This reaction is valid for preparation of primary amines only, and it gives good yields of primary amines uncontaminated with other amines.
Hofmann elimination of quaternary ammonium salts
[edit | edit source]- R3N+CH2CH2R' + OH- → R3N + H2C=CHR' + H2O
- Quaternary ammonium salts, upon treatment with a strong base, undergo the Hofmann Elimination.
Reduction of nitriles and amides
[edit | edit source]- Nitriles are reduced to amines using hydrogen in the presence of a nickel catalyst, although acidic or alkaline conditions should be avoided to avoid the possible hydrolysis of the -CN group.
- LiAlH4 is more commonly employed for the reduction of nitriles on the laboratory scale.
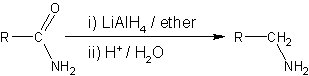
- Similarly, LiAlH4 reduces amides to amines.
Nucleophilic substitution of haloalkanes
[edit | edit source]Primary amines can also be synthesized by alkylation of ammonia. Haloalkanes react with amines to give a corresponding alkyl-substituted amine, with the release of a halogen acid. Such reactions, which are most useful for alkyl iodides and bromides, are rarely employed because the degree of alkylation is difficult to control. If the reacting amine is tertiary, a quaternary ammonium cation results. Many quaternary ammonium salts can be prepared by this route with diverse R groups and many halide and pseudohalide anions.

Properties
[edit | edit source]Types of Amines
[edit | edit source]Amines can be either primary, secondary or tertiary, depending on the number of carbon-containing groups that are attached to them. If there is only one carbon-containing group (such as in the molecule CH3NH2) then that amine is considered primary. Two carbon-containing groups makes an amine secondary, and three groups makes it tertiary. Utilizing the lone electron pair of nitrogen, it is sometimes energetically favored to use the nitrogen as a nucleophile and thus bind a fourth carbon-containing group to the amine. In this case, it could be called a quaternary ammonium ion.
Primary Amine:
|
Secondary Amine: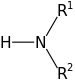
|
Tertiary Amine:
|
An organic compound with multiple amine groups is called a diamine, triamine, tetraamine and so forth, based on the number of amine groups (also called amino groups) attached to the molecule. The chemical formula for methylene diamine (also called diaminomethane), for example, would be as follows: H2N-CH2-NH2
Aromatic amines
[edit | edit source]Aromatic amines have the nitrogen atom directly connected to an aromatic ring structure. Due to its electron withdrawing properties, the aromatic ring greatly decreases the basicity of the amine - and this effect can be either strengthened or offset depending on what substituents are on the ring and on the nitrogen. The presence of the lone electron pair from the nitrogen has the opposite effect on the aromatic ring itself; because the nitrogen atom can "loan" electron density to the ring, the ring itself becomes much more reactive to other types of chemistry.
Naming conventions
[edit | edit source]For primary amines, where the amine is not the principal characteristic group, the prefix "amino-" is used. For example: 4-aminobenzoic acid where the carboxylic acid is the principal characteristic. Otherwise, the suffix "-amine" is used with with either the parent hybride or the R group substituent name. Example: ethanamine or ethylamine. Alternatively, the suffix "-azane" can be appended to the R group substituent name: Example: propylazane.
For secondary, tertiary, and quarternary amines, the naming convention is a bit different, but the suffixes are the same. For symmetrical amines, the "di" or "tri" prefix is used depending on whether there are 2 or 3 substituents. For example, dipropylamine is a secondary amine, and triphenylamine is a tertiary amine. For asymmetric amines, the parent chain gets the "-amine" suffix. This name is then prefixed with "N-" (indicating the nitrogen bond) and the substituent group name, for each substituent, using alphabetic order for tertiary amides. For example, N-ethyl-N-methyl-propylamine, not N-methyl-N-ethyl-propylamine.
To sum up:
- as prefix: "amino-"
- as suffix: "-amine"
- the prefix "N-" shows substitution on the nitrogen atom (in the case of secondary, tertiary and quaternary amines)
Systematic names for some common amines:
 methylamine |
- Primary amines: ethanamine or ethylamine.
- Secondary amines: dimethylamine
- Tertiary amines: trimethylamine
Physical properties
[edit | edit source]As one might readily guess, the inclusion of a heteroatom such as nitrogen in otherwise exclusively carbon and hydrogen molecules has quite an effect on the properties of amines as compared to alkanes.
General properties
[edit | edit source]Hydrogen bonding significantly influences the properties of primary and secondary amines as well as the protonated derivatives of all amines. Thus the boiling point of amines is higher than those for the corresponding phosphines (compounds containing phosphorus), but generally lower than the corresponding alcohols. Alcohols, or alkanols, resemble amines but feature an -OH group in place of NR2. Since oxygen is more electronegative than nitrogen, RO-H is typically more acidic than the related R2N-H compound.
Methyl, dimethyl, trimethyl, and ethyl amines are gases under standard conditions. Most common alkyl amines are liquids, and high molecular weight amines are, quite naturally, solids at standard temperatures. Additionally, gaseous amines possess a characteristic ammonia smell, while liquid amines have a distinctive "fishy" smell.
Most aliphatic amines display some solubility in water, reflecting their ability to form hydrogen bonds. Solubility decreases relatively proportionally with the increase in the number of carbon atoms in the molecule - especially when the carbon atom number is greater than six. Aliphatic amines also display significant solubility in organic solvents, especially in polar organic solvents. Primary amines react readily with ketone compounds (such as acetone), however, and most amines are incompatible with chloroform and also with carbon tetrachloride as solvent solutions.
Aromatic amines have their lone pair electrons conjugated ("shared") into the benzene ring, so their tendency to engage in hydrogen bonding is somewhat diminished. The boiling points of these molecules are therefore usually somewhat higher than other, smaller amines due to their typically larger size. They also often have relatively diminished solubility in water, although they retain their solubility in other organic solvents.
Aromatically conjugated amines are often quite toxic and have the potential to be easily absorbed through the skin, so should always be treated as "hazardous".

Chirality
[edit | edit source]Tertiary amines of the type NHRR' and NRR'R" are not chiral: although the nitrogen atom bears four distinct substituents counting the lone pair, the lone pair of electrons can "flip" across the nitrogen atom and invert the other molecules. The energy barrier for just such a Walden inversion of the stereocenter with a lone pair of electrons is relatively low, e.g. ~7 kcal/mol for a trialkylamine, therefore it is difficult to obtain reliably chiral products using tertiary amines. Because of this low barrier, amines such as NHRR' cannot be resolved optically and NRR'R" can only be resolved when the R, R', and R" groups are constrained in cyclic structures. Quaternary amine structures, e.g. H3C-N+-RR'R", are chiral and are readily optically resolved.
Properties as bases
[edit | edit source]Like ammonia, amines act as bases and are reasonably strong (see the provided table for some examples of conjugate acid Ka values). The basicity of amines varies by molecule, and it largely depends on:
- The availability of the lone pair of electrons from nitrogen
- The electronic properties of the attached substituent groups (e.g., alkyl groups enhance the basicity, aryl groups diminish it, etc.)
- The degree of solvation of the protonated amine, which depends mostly on the solvent used in the reaction
The nitrogen atom of a typical amine features a lone electron pair which can bind a hydrogen ion (H+) in order to form an ammonium ion -- R3NH+. The water solubility of simple amines is largely due to the capability for hydrogen bonding that can occur between protons on the water molecules and these lone pairs of electrons.
---
- Inductive effect of alkyl groups
| Ions of compound | Kb |
|---|---|
| ammonia NH3 | 1.8·10-5 M |
| methylamine CH3NH2 | 4.4·10-4 M |
| propylamine CH3CH2CH2NH2 | 4.7·10-4 M |
| 2-propylamine (CH3)2CHNH2 | 5.3·10-4 M |
| diethylamine (CH3)2NH2 | 9.6·10-4 M |
+I effect of alkyl groups raises the energy of the lone pair of electrons, thus elevating the basicity.
- Mesomeric effect of aromatic systems
| Ions of compound | Kb |
|---|---|
| ammonia NH3 | 1.8·10-5 M |
| aniline C6H5NH2 | 3.8·10-10 M |
| 4-methylphenylamine 4-CH3C6H4NH2 | 1.2·10-9 M |
+M effect of aromatic ring delocalizes the lone pair electron into the ring, resulting in decreased basicity.
The degree of protonation of protonated amines:
| Ions of compound | Maximum number of H-bond |
|---|---|
| NH4+ | 4 Very Soluble in H2O |
| RNH3+ | 3 |
| R2NH2+ | 2 |
| R3NH+ | 1 Least Soluble in H2O |
Reactions
[edit | edit source]Amide formation
[edit | edit source]Acyl chlorides and acid anhydrides react with primary and secondary amines without the presence of heat to form amides. Tertiary amines cannot be acylated due to the absence of a replaceable hydrogen atom. With the much less active benzoyl chloride, acylation can still be performed by the use of excess aqueous base to facilitate the reaction.
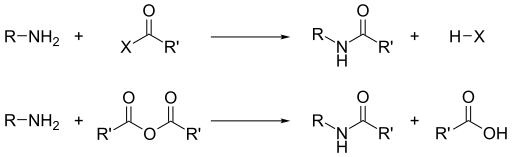
Salt formation
[edit | edit source]Because amines are basic, they neutralize carboxylic acids to form the corresponding ammonium carboxylate salts. Upon heating to 200°C, the primary and secondary amine salts dehydrate to form the corresponding amides.
Neutralization
[edit | edit source]Amines R3N react with strong acids such as hydroiodic acid (HI), hydrobromic acid (HBr) and hydrochloric acid (HCl) to give ammonium salts R3NH+.
Reaction with nitrous acid
[edit | edit source]Nitrous acid with the chemical formula HNO2 is unstable. Usually it is produced indirectly in a mixture of NaNO2 and a strong acid such as HCl or H2SO4 in dilute concentration, so that the H+ ions will associate with the NO2- ions in solution.
Primary aliphatic amines with nitrous acid give very unstable diazonium salts which spontaneously decompose by losing N2 to form a carbenium ion. The carbenium ion goes on to produce a mixture of alkenes, alkanols or alkyl halides, with alkanols as the major product. This reaction is of little synthetic importance because the diazonium salt formed is too unstable, even under quite cold conditions.
- NaNO2 + HCl → HNO2 + NaCl

- Primary aromatic amines, such as aniline (phenylamine) forms a more stable diazonium ion at 0–5°C. Above 5°C, it will decompose to give phenol and N2. Diazonium salts can be isolated in the crystalline form but are usually used in solution and immediately after preparation, due to rapid decomposition on standing even with little ambient heat. Solid diazonium salts can be explosive on shock or on mild warming.

Reactions with ketones and aldehydes
[edit | edit source]- Primary amines react with carbonyl compounds to form imines. Specifically, aldehydes become aldimines, and ketones become ketimines. In the case of formaldehyde (R' = H), the imine products are typically cyclic trimers.
- RNH2 + R'2C=O → R'2C=NR + H2O
- Secondary amines react with ketones and aldehydes to form enamines. An enamine contains a C=C double bond, where the second C is singly bonded to N as part of an amine ligand.
- R2NH + R'(R"CH2)C=O → R"CH=C(NR2)R' + H2O
Use of amines
[edit | edit source]As dyes
[edit | edit source]Primary aromatic amines are used as a starting material for the manufacture of azo dyes. They react with nitrous(II) acid to form diazonium salt which can undergo a coupling reaction in order to form an azo compound. As azo compounds are highly coloured, they are widely used in dyeing industries. Examples include:
- Methyl orange
- Direct brown 138
- Sunset yellow FCF
- Ponceau
As drugs
[edit | edit source]- Chlorpheniramine is an antihistamine the helps to relief allergic disorders due to cold, hay fever, itchy skin, insect bites and stings.
- Diphenhydramine is the common antihistamine, benadryl.
- Chlorpromazine is a tranquillizer that sedates without inducing sleep. It is used to relieve anxiety, excitement, restlessness or even mental disorder.
- Acetaminophen is also known as paracetamol or p-acetaminophenol, an analgesic that relieves pains such as headaches. It is believed to be less corrosive to the stomach and is an alternative to aspirin.
See also
[edit | edit source]- IUPAC nomenclature for the official naming rules for amines.

