A-level Chemistry/OCR (Salters)/Reactions of arenes

Reactions of arenes is the title of Section 12.4 in Chemical Ideas.
Introduction[edit | edit source]
Arenes are much more stable and less reactive than alkenes. Unlike alkenes, arenes do not undergo electrophilic addition reactions because doing so would destroy the arene's aromaticity (stability due to electron delocalisation in a ring).
The characteristic reactions of arenes are entirely different. They tend to undergo electrophilic substitution, which involves an electrophile taking the place of a hydrogen atom on the arene's benzene ring.
Bromination of benzene[edit | edit source]

Overview[edit | edit source]
Mechanism[edit | edit source]
Chlorination[edit | edit source]
Overview[edit | edit source]
Anhydrous conditions are essential whenever aluminium chloride, AlCl3, is used — upon contact with water, an explosive reaction may occur, releasing corrosive hydrogen chloride gas.
Mechanism[edit | edit source]
Friedel-Crafts reactions[edit | edit source]
Friedel-Crafts alkylation[edit | edit source]
Overview[edit | edit source]
Mechanism[edit | edit source]
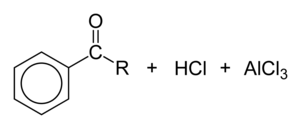
Friedel-Crafts acylation[edit | edit source]
Overview[edit | edit source]
Mechanism[edit | edit source]
The electrophile at the centre of Friedel-Crafts acylation is the acylium ion, [RCO]+. Its carbon atom carries a large partial positive charge and is highly electrophilic, allowing it to react with very unreactive arenes such as benzene. Bonding in the acylium ion is best described as a resonance hybrid of two canonical forms:
Nitration[edit | edit source]
Overview[edit | edit source]
Arenes react with NO2+, a highly electrophilic linear cation called the nitronium ion. As a result, a nitro group, RNO2, replaces a hydrogen atom on the arene's benzene ring.
Because NO2+ is highly reactive, it is not readily available as a salt. It must be generated in situ (in the reaction vessel) by the reaction of concentrated sulfuric acid with concentrated nitric acid, which together are called a nitrating mixture.
- HNO3 + 2H2SO4 → NO2+ + 2HSO4− + H3O+
For every mole of benzene nitrated, one mole of nitric acid is consumed, so nitric acid is a reactant. By contrast, sulfuric acid is regenerated once nitration has occurred, making it a catalyst.

In order to limit the number of nitro groups added, the temperature must be kept below 55 °C. Above this temperature, dinitro and trinitro compounds are formed, which are often dangerously unstable and prone to explosion. An example is TNT (trinitrotoluene, a trivial name for 2,4,6-trinitromethylbenzene).
Mechanism[edit | edit source]
Reduction of nitroarenes to arylamines[edit | edit source]
Tin in concentrated hydrochloric acid is an effective catalyst for the reduction of a nitroarene to an arylamine.
Sulfonation[edit | edit source]

Overview[edit | edit source]
Mechanism[edit | edit source]
Concentrated sulfuric acid contains some sulfur trioxide, SO3, which is thought to be the electrophile involved in the sulfonation of arenes. SO3 has a large positive partial charge on its central sulfur atom, which is electrophilic enough to attract delocalised electrons from a benzene ring.
Hydrogenation[edit | edit source]
Benzene is very difficult to hydrogenate because doing so dispenses with the stable aromatic system of delocalised electrons. To hydrogenate benzene requires a highly active catalyst and conditions of high temperature and pressure.
The catalyst is based on very finely divided nickel and is called Raney nickel. The conditions used in industry are a pressure of 30 atm and a temperature of 300 °C. Three moles of dihydrogen, H2, are required to hydrogenate one mole of benzene.
Summary[edit | edit source]
With the exception of hydrogenation, the reactions of arenes presented here are all electrophilic substitution reactions. Arenes, especially benzene, have an exceptionally stable and unreactive aromatic system of delocalised electrons. Consequently, arenes only react with the most reactive of electrophiles and special catalysts are required to generate the highly electrophilic species required, such as Br+, NO2+ and R+.

Once a suitably electrophilic species, E+, has been generated, it will react rapidly with benzene. A highly unstable intermediate (containing a tetrahedral carbon) is formed, which significantly disrupts the delocalisation of electrons in the ring. The intermediate immediately releases a proton in order to regain a fully aromatic ring and hence decrease its energy considerably.
A more detailed look at the mechanism shows that a hydrogen atom from benzene is replaced by the electrophile.