Science: An Elementary Teacher’s Guide/Building Blocks of Matter
Atoms, Elements, Molecules, Compounds, and Mixtures[edit | edit source]
Matter is any substance that has mass and takes up space by having volume. All everyday objects that we can touch and see are ultimately composed of atoms, which are themselves made up of interacting subatomic particles, such as protons, neutrons, and electrons (and these are made of even smaller particles!). Matter generally includes atoms and anything made up of the infinite variety of combinations of atoms attached to each other as molecules. Single atoms are far too small to see or work with, but if we have trillions of atoms of all the same type (such as a sample of pure gold, or pure helium), we can study the properties of that element. We have learned about the properties of different elements and organized them into a "Periodic Table of the Elements" (see section below).
Two or more atoms bound to each other are called a molecule, and we will later discuss how they bind to each other. A molecule can have very different properties than the atoms it is made from (Hydrogen is a gas, and so is Oxygen, but if you combine two Hydrogen atoms with one Oxygen atom, you get H2O (water), which has very different properties than the elements it is made from).
A compound is many molecules of the same type. Hence, a glass of water is a compound of water molecules, and a spoonful of sugar is a compound of sugar molecules. An egg shell is mainly calcium carbonate compound (many molecules of calcium carbonate molecules joined together).
A mixture is simply a mix of different things! So, if you add sugar and water together, now you have a mixture instead of two compounds. For liquid mixtures we can use the word solution instead, which consists of a solvent (the liquid part, such as water) and a solute (the solid part that gets dissolved into the solvent--the sugar, for instance).
The air around you is a mixture of different gas molecules (Nitrogen, Oxygen, Carbon dioxide, water vapor, and others). It's easy to combine compounds to get a mixture; it is trickier to separate compounds out of a mixture but it can be done: for instance you could take your sugar water mixture, boil it, collect the water vapor, and you would again have a compound of pure water, since the sugar would be left behind (and if you let it dry completely you should be left with a pure sugar compound).
Atomic Properties: Protons, Neutrons, and Electrons[edit | edit source]
Every atom is composed of a nucleus and one or more electrons zipping very quickly around the nucleus. The nucleus is made of one or more protons and typically a similar number of neutrons (the exception is Hydrogen, which has only 1 proton and no neutrons). The protons have a positive electric charge and each proton has a mass of 1 atomic mass unit (AMU); the electrons have a negative electric charge and almost no mass (5/10,000ths of an AMU), and the neutrons have no electric charge and a mass similar to the protons. If the number of protons and electrons are equal, that atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively, and it is called an ion. The number of protons defines what type of atom it is: a Hydrogen always has 1 proton per atom and Carbon always has 6 protons per atom. The number of protons can only be changed in nuclear reactions, and if the proton number is changed then that atom has become a different element. In contrast, the number of electrons can change quite easily depending on the atom and which other types of atoms it gets exposed to. Since the electrons are on the outside of the atom, they are what interacts with other atoms, and it is the electrons that are responsible for most of the chemical properties of an element. The number of neutrons is not likely to change, and when it does change the chemical properties of that atom stay the same (atoms of the same element that have different numbers of neutrons are called isotopes, and these can be radioactive and have various possible uses). An atom's mass is 99.95% in the nucleus--the electrons are incredibly tiny when compared to the protons and neutrons and contribute almost no mass. In the Periodic Table of the Elements, the Atomic Mass is shown on the bottom left of each square and is the sum of all the protons, neutrons, and electrons.
The arrangement of particles within the atom is interesting: the protons and neutrons are tightly packed together in a small region called the nucleus (don't confuse it with the nucleus of a cell--it is the same word, but not the same thing). Since positive charges repel each other, it is strange that protons can be held together so tightly in the nucleus of an atom (along with the neutrons). There is much still to learn about "the strong nuclear force" that keeps nuclei together, but for our class we will simply say it is the strongest known force in the universe! It is also the force that must be broken during a nuclear reaction! The electrons are held to the atom by the "electromagnetic force," which is much more understood (and is responsible for magnetism and electricity) and is much weaker. The distance between the electrons and the nucleus is actually quite large: if the nucleus of an atom was the size of a golfball, and you set that golfball in the middle of a football stadium, the electrons would be like tiny specks of sand traveling at incredible speeds around and around the edge of the football stadium! In other words, every atom is mostly EMPTY SPACE! If you were to take away all the empty space from all the atoms in your body, you would be able to fit your entire body into the ink at the top of this letter "i!" (and amazingly, that tiny dot would still weigh the same amount that you do, because it would still contain the mass of all your protons and neutrons!).
We actually can never know exactly where the electrons are since they are so tiny and they are moving so fast, but they tend to stay in "electron orbitals," which are like different clouds around the nucleus. Different atoms, with different numbers of electrons, have different shaped clouds. The outermost cloud (or "shell") of electrons is called the "valence shell," and the electrons in that shell are the "valence electrons." These are the electrons that interact with the valence electrons of other atoms, and the electrons might get transferred from one atom to another, or shared between two atoms (chemical bonds will be discussed elsewhere), or simply repel each other. When you pound your fist against a desk it seems that both your fist and the desk are very solid, but really you are just experiencing the strong repelling force of trillions of electrons buzzing around the atoms of your hand that are repelling the trillions of electrons buzzing around the atoms of your desk.
Scale of the Universe[edit | edit source]
Atoms are extremely small; typical sizes are around 100 picometers (a ten-billionth of a meter). Not only is this way too small for us to see, but it is way too small for us to see with a microscope! To help you visualize this, please explore this "Scale of the Universe" animation (requires Flash to run; if you cannot access it, then watch this video). Pay attention to the circles, as each time you zoom in or out past a circle it represents 1000 times smaller or 1000 times larger! Zoom in until you find atoms, then zoom in further to find protons, neutrons, and eventually electrons. If you keep zooming in you will find all sorts of strange particles, because even the protons and neutrons are made of other things. If you go the other direction you will see larger and larger object, planets, galaxies, and so forth. We will save the very big things for a different chapter!
Metric System[edit | edit source]
The metric system is so useful that after its invention it was eventually adopted by almost every nation in the world (with the United States being the exception!). Even in the U.S., all scientists use the metric system. Why? Well, the older, Imperial system is quite inconsistent: there are 12 inches in 1 foot, 3 feet in 1 yard, but 16 ounces in one pound, and 8 fluid ounces in one cup. . . it can be very confusing. It was practical in the old days--an inch is about the size of the end of your thumb. One foot is about the size of (surprise) a foot. The metric system is all based on multiplying and dividing by tens, hundreds, and thousands, so it is much easier to figure out that 453 centimeters is 4.53 meters than it is to figure out that 453 inches is 37 feet, 9 inches. The other nice thing with the metric system is there are standard prefixes that are used, whether you are talking about length, weight, volume, or anything else.
To understand the metric system better, just know that for whatever measure you are talking about, there is a standard unit. For mass it is the gram, for volume it is the liter, for computer data it is the byte, for length it is the meter. Once you know the standard unit, then you can use prefixes as an easy way to discuss things that are much smaller or much larger than the standard unit. For example, I could measure my pencil in meters, but it is a bit awkward to call it 0.12 meters when I could call it 12 centimeters.
One thing that can make it tricky for people to think about these prefixes is that they may be uncomfortable with using scientific notation, which involves exponents. 100 = 10 X 10 = 102. 100,000 = 10 X 10 X 10 X 10 X 10, or 105. It may be helpful to notice that with 105 there is a 1 followed by 5 zeroes. That is simple enough, but it is less simple sometimes to think about things that are smaller than 10. For instance, 1/1000th = 10-3. If you divide 1 by 1000, you will get the answer 0.001 on your calculator. It may help to realize that with 10-3 you need to move the decimal 3 places to the right to get "1," or you might prefer to think, "I write 1, then I move the decimal 3 places to the left." With the metric system, the most important prefixes are when we multiply or divide by 1,000. For instance, a meter divided by 1,000 = 1 millimeter ("milli" means "one thousandth"), written in shorthand as 1 mm. If we multiply by 1,000 we get 1 kilometer ("kilo" means "one thousand"), written in shorthand as 1 km. We could do the same with volume to get 1 mL (milliliter) or 1 kL (1 kiloliter). We could divide a single millimeter into 1,000 smaller pieces. Each of those would be 1/1000th of a millimeter, or 1/1,000,000th (a millionth) of a meter. For this we use the prefix "micro," so we would say 1 micrometer (it is represented in shorthand with the Greek lowercase mu, which looks like μ (so 1 micrometer is written 1 μm). And if we divide 1 micrometer into 1,000 pieces we get 1 nanometer!

Solids, Liquids, Gases[edit | edit source]
Outer Space is mostly empty, with vast distances of nothingness between the "non-nothing" galaxies (of which there are hundreds of billions!). But wherever there are galaxies, there will also be planets consisting of matter that was mostly made in stars. For our purposes, matter consists of things made of atoms (therefore does not include photons of light, or sound waves, or heat or other forms of energy, even though matter and energy are linked together by Einstein's equation).

Matter is found in three primary states: solid, liquid, and gas. Some substances, including water, can exist in all three states. Which state it is in is a function of variables like temperature and pressure. Many elements are solid at room temperature and require extreme heat to melt into a liquid (for example Iron). Other elements are a gas at room temperature (such as Nitrogen or Oxygen) and require extreme cold before they will condense into a liquid (liquid nitrogen boils into a gas at the extremely low temperature of -196 degrees Celsius (-321 degrees Fahrenheit).
In a solid, such as a rock or a desk or a glass window, the atoms within the solid are still vibrating and jiggling, but they cannot move about freely because they are found strongly to the other atoms around themselves. Different solids have different properties, depending on what types of atoms are joined together. Hence, a smooth river rock being dull and smooth metal being shiny is a function of the molecules themselves.
In a liquid, the molecules are moving freely, bumping into each other in random patterns. Even in a glass of water that has been sitting on a table for an hour, perfectly still, the water molecules within are in constant motion. You can prove this to yourself by putting in a drop of food coloring--instead of staying in one place the color quickly spreads, thanks to the constant motion of the water molecules. Heat is actually a measure of how quickly molecules are moving or vibrating. Hence, if you put one glass of water in the refrigerator, and heat up another glass of water in a microwave, then apply a drop of food coloring to both, you will be able to see the effect of different speeds of molecular movement.
In a gas, the molecules are more spread out than in a liquid. In fact, whatever size or shape of container, the gas molecules will spread out evenly and occupy the container with even pressure throughout. Unlike a liquid or solid, a gas is easily compressed. As you compress a gas the molecules are forced closer together. The more a gas is compressed, the greater the pressure pushing back on the container (generally measured in pounds per square inch, or PSI). This pressure is useful for tires (a typical car tire is inflated to about 35 PSI), or for spraying off your electronics with a can of compressed air, or for being able to carry large quantities of air on your back for SCUBA diving. As a gas decompresses the pressure drops and so does the temperature. You may have felt a can of compressed air getting very cold after it has been sprayed for several seconds. This changing temperature based on pressure is used in home and car cooling systems. An air-conditioner has a compressor to heat gases, and this heat is released to the environment, then the gas is allowed to expand and cool some pipes and air is blown over these pipes to bring a current of cool air into the home or vehicle.
The Birth of Elements[edit | edit source]
The light and heat that comes from every star is a product of nuclear fusion reactions. Our sun generates its energy by nuclear fusion of hydrogen nuclei into helium. In its core, the Sun fuses 620 million metric tons of hydrogen each second. In the fusion of four hydrogen nuclei to form helium, 0.7% of the mass is carried away in the form of kinetic energy of an alpha particle or other forms of energy, such as electromagnetic radiation, following Einstein's infamous equation, E = mc2 (Energy is equal to mass multiplied by the speed of light, squared). Energy released in nuclear reactions is much larger than in chemical reactions, because the binding energy that holds a nucleus together is much greater than the energy that holds electrons to a nucleus (remember, the strong nuclear force is the strongest force we know of). To put this into perspective, if you could fuse the hydrogen atoms in a simple glass of water, you would have enough energy to drive your car for 10,000 miles! So there is tremendous energy in all the matter around us, but most of the energy is unavailable because of how difficult it is to take apart atomic nuclei (fission) or smash them together (fusion). Stars can carry out fusion because they are so massive (see Scale of the Universe) that the gravity creates intense pressure and heat in the core of the star. Hydrogen is the smallest atom (just 1 proton), and is by far the most common element in the universe. Hydrogen is the basic fuel of the stars, at least while they are young ("young" for a star can be several billion years). Helium has 2 protons, and 2 neutrons (remember that Hydrogen has no neutrons). One Helium is about 0.7% less than the mass of four Hydrogen atoms (because a small portion of the mass was converted to energy during the fusion reaction). If 2 more Hydrogens were to fuse with Helium they could make an atom with 3 protons and 3 neutrons, which would be Lithium. In other words, an element is defined by the number of protons it has, and all the elements heavier than Hydrogen have been created in stars. It is amazing to think that all the solid objects on our planet (including you) are made of atoms that were birthed in distant stars! The heaviest elements are synthesized by fusion that occurs as a more massive star undergoes a violent supernova at the end of its life (as a star ages it uses up its lighter elements and moves on to fusion of heavier and heavier elements--the ashes of one round of burning becomes the fuel for the next round). The supernova star then explodes and the heavier elements are thrown like cosmic dust in all directions. Some of the dust and crystals start to clump together in giant cosmic clouds. In the center of that cloud a new star may form, and planets may form as the debris clumps together more and more. Our Earth is believed to have been formed, along with the other planets of our solar system, over a period of 10-20 million years about 4.54 billion years ago.
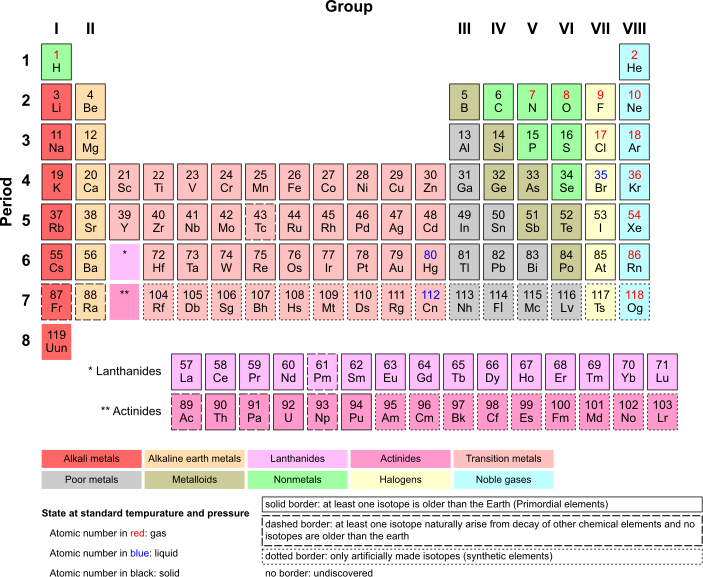
How to Organize Elements: The Periodic Table[edit | edit source]
We live on a planet that is made of iron, nickel, carbon, silicon, and many other elements. Some elements are common (such as Oxygen) while others are rare (such as gold). As chemists have studied the elements they have organized their knowledge in various ways. One of the most useful ways to organize the elements is called the Periodic Table of the Elements. In this table the atoms are ordered sequentially based on the number of protons: Hydrogen has an Atomic Number of 1 because it has 1 proton, Helium 2 because it has 2 protons, Silver has an atomic number of 47 because it has 47 protons. You get the idea. As you move left to right and top to bottom, the elements are getting heavier and heavier. The Atomic Number only tells the number of protons. The Atomic Mass is slightly different--it includes the combined weight of the protons plus the neutrons (technically it includes the electrons, but their weight is negligible). Carbon has 6 protons and 6 neutrons, and an atomic mass of 12.011. The reason it is not 12.000 is that sometimes a carbon atom will have an extra neutron (so it is called Carbon13), or two extra neutrons (called Carbon14). There are very few C13 or C14 isotopes, but there are enough of them that they make any clump of pure carbon a tiny bit heavier than if carbon only consisted of C12 atoms. [side-note: C14 is produced by CO2 getting bombarded with ions high in the earth's atmosphere. It slowly decays at a known rate to C12. Living things take in C14 at a constant rate, and when they die they stop taking in any more. Archaeologists and paleontologists can measure the amount of C14 that is left and estimate how old something is--this is called carbon dating.]
One important thing about the Periodic Table is that the columns consist of atoms with similar chemical properties because they have similar valence electrons. The column on the left (Group 1, with H, Li, Na, K, and so forth) all have 1 electron in their outermost shell. The next column (group 2) all have 2 electrons in their outer shell. This rule kind of gets broken for the middle section of metals, for reasons we won't discuss--they mostly have 2 electrons in their outer shells. Then, in the 13th column (the Boron group) there are 3 electrons in the outer shell. Next comes the Carbon group with 4 electrons in the valence shell. Then 5, then 6, then 7 electrons in the outer shell, and finally the column on the far right--with 8 electrons in the outer shell (with the exception of Helium, which will be explained elsewhere). Why does this matter? It matters because how an element acts, chemically, is based largely on how many electrons are in the outer shell. This will be discussed more fully in the sections about chemical reactions and bonds, but for now we will say that "it's the dream of every atom to have a full outer shell of electrons." (True, atoms don't dream, but that is more interesting than stating something like "atoms achieve maximum stability if their valence shell is full"). The far right (Group 18) all have complete, full outer shells. They are called the "Noble Gases" and simply float around as individual atoms, not attracted to any other atom and not interacting chemically with anyone. As we will see, all other columns are ready to act in various ways.
In the simplified Periodic Table below you will see the one or two-letter symbol for each element, the atomic number (which refers to the number of protons per atom for that element), and the arrangement into Groups (columns) and Periods (rows). A full periodic table can be seen at this link, which gives a bit more information about each element. If you want to learn more about any particular element in a fun way, watch some of the amusing content at Periodic Videos from the University of Nottingham in England (complete with a real, crazy-haired mad scientist!).

Next Chapter: Chemical Reactions