High School Chemistry/Atomic Size
In the Periodic Table, there are a number of physical properties that are not really "similar" as it was previously defined, but are more trend-like. This means is that as you move down a group or across a period, you will see a trend-like variation in the properties. There are three specific periodic trends that we will discuss. The first lesson of this chapter is devoted to the trend in atomic size in the Periodic Table. The two following this lesson will discuss ionization energy and electron affinity. Each of these trends can be understood in terms of the electron configuration of the atoms.
The actual trends that are observed with atomic size have to do with three factors. These factors are:
- The number of protons in the nucleus (called the nuclear charge).
- The number of energy levels holding electrons (and the number of electrons in the outer energy level).
- The number of electrons held between the nucleus and its outermost electrons (called the shielding effect).
Lesson Objectives
[edit | edit source]- Define atomic radius.
- State the boundary issue with atomic size.
- Describe measurement methods for atomic size.
- Define the shielding effect.
- Describe the factors that determine the trend of atomic size.
- Describe the general trend in atomic size for groups and for periods.
- Describe the trend of atomic radii in the rows in the Periodic Table.
- Describe how the trend of atomic radii works for transition metals.
- Use the general trends to predict the relative sizes of atoms.
- Use the concept of effective nuclear charge to explain why the atomic radii of the main group elements increase when we move down a group in the periodic table
- due to complex structure of f-orbital and poor shielding of f-e(s)= lanthanoids contractions occur.
Atoms Have No Definite Boundary
[edit | edit source]The region in space occupied by the electron cloud of an atom is often thought of as a probability distribution of the electrons and therefore, there is no well-defined "outer edge" of the electron cloud. Atomic size is defined in several different ways and these different definitions often produce some variations in the measurement of atomic sizes.
Because it is so difficult to measure atomic size from the nucleus to the outermost edge of the electron cloud, chemists use other approaches to get consistent measurements of atomic sizes. One way that chemists define atomic size is by using the atomic radius. The atomic radius is one-half the distance between the centers of a homonuclear diatomic molecule (a diatomic molecule means a molecule made of exactly two atoms and homonuclear means both atoms are the same element). The figure below represents a visualization of the atomic size definition.
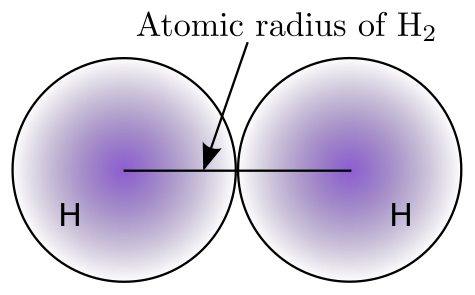
How do we measure the size of the atom? Ernest Rutherford is famous for his experiments bombarding gold foil with alpha particles. The gold foil experiment by Rutherford, first done in 1911, is of particular interest to us in this unit because it was this experiment that first gave science an approximate measurement for the size of the atom. He was able, using technology available in the early part of the 1900s, to determine quantitatively that the nucleus had an approximate size of 4×10−12 cm. The size of the atom is slightly larger, approximately 2×10−8 cm in diameter.
Atomic Size in a Column Increases from Top to Bottom
[edit | edit source]Let's now look at the atomic radii or the size of the atom from the top of a family or group to the bottom. Take, for example, the Group 1 metals. Each atom in this family (and all other main group families) has the same number of electrons in the outer energy level as all the other atoms of that family. Each row (period) in the periodic table represents another added energy level. When we first learned about principal energy levels, we learned that each new energy level was larger than the one before. Energy level 2 is larger than energy level 1, energy level 3 is larger than energy level 2, and so on. Therefore, as we move down the Periodic Table from period to period, each successive period represents the addition of a larger energy level. It becomes apparent that as we move downward through a family of elements, that each new atom has added another energy level and will, therefore, be larger.

One other contributing factor to atomic size is something called the shielding effect. The protons in the nucleus attract the valence electrons in the outer energy level because of opposite electrostatic charges. The strength of this attraction depends on the size of the charges, the distance between the charges, and the number of electrons in-between the nucleus and the valence electrons. The core electrons shield the valence electrons from the nucleus. The presence of the core electrons weakens the attraction between the nucleus and the valence electrons. This weakening of the attraction is called the shielding effect. The amount of shielding depends on the number of electrons between the nucleus and the valence electrons. The strength with which the nucleus pulls on the valence electrons can pull the valence shell in tighter when the attraction is strong and not so tight when the attraction is weakened. The more shielding that occurs, the further the valence shell can spread out.
| Element | # of protons | Electron Configuration | # of energy levels |
|---|---|---|---|
| Li | 3 | [Ne]2s1 | 2 |
| Na | 11 | [He]3s1 | 3 |
| K | 19 | [Ar]4s1 | 4 |
| Rb | 37 | [Kr] 5s1 | 5 |
| Cs | 55 | [Xe]6s1 | 6 |
For example, if you are looking at the element sodium, it has the electron configuration:
- Na: 1s22s22p63s1
The outer energy level is n = 3 and there is one valence electron but the attraction between this lone valence electron and the nucleus that has those 11 protons is shielded by the other 10 inner (or core) electrons.
When we compare an atom of sodium to one of cesium, we notice that the number of protons increases as well as the number of energy levels occupied by electrons. There are also many more electrons between the outer electron and the nucleus, thereby shielding the attraction of the nucleus.
- Cs: 1s22s22p63s23p64s23d104p65s24d105p66s1
The outermost electron, 6s1, therefore, is held very loosely. In other words, because of shielding, the nucleus has less control over this 6s1 electron than it does over a 3s1 electron. The result of all of this is that the atom's size will be larger. Table 10.2 gives the values for the atomic radii for the Group 1 metals plus a visual representation to appreciate the size change in a group in the periodic table (measurement units for atomic radii are picometers [pm] or 1×10−12 meters).
| Element | Atomic Radii | Visual |
|---|---|---|
| Li | 123 pm | 
|
| Na | 157 pm | 
|
| K | 203 pm | 
|
| Rb | 216 pm | 
|
| Cs | 235 pm | 
|
What is true for the Group 1 metals is true for all of the groups, or families, across the periodic table. As you move downward in the periodic table through a family group, the size of the atoms increases. For instance, the atoms that are the largest in the halogen family are bromine and iodine (since astatine is radioactive and only exists for short periods of time, we won't include it in the discussion).
|
Sample Question Which of the following is larger? Explain.
Solution:
|
As noted earlier for the main group metals, the outermost energy level in the electron configuration is indicated by the period number. So the element magnesium (Z = 12), is in Period 3, Group 2. According to this, we can say that there are 3 energy levels with 2 electrons in the outermost energy level. Let's look at the electron configuration for magnesium.
- Mg: 1s22s22p63s2
Moving from magnesium to strontium, strontium is in the 5th period of Group 2. This means that there are two electrons in the valence energy level. Strontium also has electrons occupying five energy levels.
- Sr: 11s22s22p63s23p64s23d104p65s2
You can imagine that with the increase in the number of energy levels, the size of the atom must increase. The increase in the number of energy levels in the electron cloud takes up more space.
Therefore, the trend within a group or family on the periodic table is that the atomic size increases with increased number of energy levels. The Periodic Table below shows the trend of atomic size for groups. The arrow indicates the direction of the increase.

Atomic Size in a Period Decreases from Left to Right
[edit | edit source]In order to determine the trend for the periods, we need to look at the number of protons (nuclear charge), the number of energy levels, and the shielding effect. For a row in the Periodic Table, the atomic number still increases (as it did for the groups) and thus the number of protons would increase. When we examine the energy levels for Period 2, we find that the outermost energy level does not change as we increase the number of electrons. In Period 2, each additional electron goes into the second energy level. So the number of energy levels does not go up. As we move from left to right across a period, the number of electrons in the outer energy level increases but it is the same outer energy level. Table 10.3 shows the electron configuration for the elements in Period 2.
| Element | # of protons | Electron Configuration |
|---|---|---|
| Lithium (Li) | 3 | 1s22s1 |
| Beryllium (Be) | 4 | 1s22s2 |
| Boron (B) | 5 | 1s22s22p1 |
| Carbon (C) | 6 | 1s22s22p2 |
| Nitrogen (N) | 7 | 1s22s22p3 |
| Oxygen (O) | 8 | 1s22s22p4 |
| Fluorine (F) | 9 | 1s22s22p5 |
Looking at the elements in Period 2, the number of protons increases from lithium with three protons, to fluorine with nine protons. Therefore, the nuclear charge increases across a period. Meanwhile, the number of energy levels occupied by electrons remains the same. The numbers of electrons in the outermost energy level increases from left to right along a period but how will this affect the radius? We know that every one of the elements in row #2 has two electrons in their inner energy level (2 core electrons). The core electrons shield the outer electrons from the charge of the nucleus. With lithium, there are two core electrons and one valence electron so those two core electrons will shield the one outer electron. In beryllium, there are four protons being shielded by the 1s2 electrons. With the increasing number of protons attracting the outer electrons and the same shielding from the core electrons, the valence electrons are pulled closer to the nucleus, making the atom smaller.
In group 17, the first element in the group is fluorine. With fluorine, there are 9 protons and 9 electrons. The electronic configuration is 1s22s22p5. However, there are still the same core electrons as with lithium and beryllium, that is, the 1s2 electrons. Since there are more protons, there is an increase in the nuclear charge. With an increase in nuclear charge, there is an increase in the pull between the protons and the outer level, pulling the outer electrons toward the nucleus. The amount of shielding from the nucleus does not increase because the number of core electrons remains the same (1s2 for this period). The net result is that the atomic size decreases going across the row. In Table 10.4, the values are shown for the atomic radii for the row starting at lithium and ending with fluorine plus a visual representation to appreciate the size change in a group in the Periodic Table.
| Element | Atomic Radii | Visual |
|---|---|---|
| Li | 123 pm | 
|
| Be | 111 pm | 
|
| B | 86 pm | 
|
| C | 77 pm | 
|
| N | 74 pm | 
|
| O | 73 pm | 
|
| F | 72 pm | 
|
Let's add this new trend to the Periodic Table. Look at the diagram below of our new "periodic trend table". In the diagram you will notice that the trend arrow for the period shows the atomic radii increase going from right to left, which is the same as decreasing from left to right.

Considering all the information about atomic size, you will recognize that the largest atom on the periodic table is all the way to the left and all the way to the bottom, francium, #87, and the smallest atom is all the way to the right and all the way to the top, helium, #2.
The fact that the atoms get larger as you move downward in a family is probably exactly what you expected before you even read this section, but the fact that the atoms get smaller as you move to the right across a period is most likely a big surprise. Make sure you understand this trend and the reasons for it.
For the Transition Elements, the Trend is Less Systematic
[edit | edit source]A general trend for atomic radii in the Periodic Table would look similar to that found in the diagram below. The elements with the smallest atomic radii are to the upper right; those with the largest atomic radii are to the lower left.

Until now, we have worked solely with the main group metals. Let's look at our three factors and see how these factors fit the transition metal series. Looking at the first row of the transition metals, the 3d row, Table 10.5 shows the number of protons in each of the 10 elements in this row. The number of protons is increasing so the nuclear charge is increasing.
| Element | # of protons | Electron Configuration |
|---|---|---|
| Scandium (Sc) | 21 | [Ar]3d14s2 |
| Titanium (Ti) | 22 | [Ar]3d24s2 |
| Vanadium (V) | 23 | [Ar]3d34s2 |
| Chromium (Cr) | 24 | [Ar]3d54s1 |
| Manganese (Mn) | 25 | [Ar]3d54s2 |
| Iron (Fe) | 26 | [Ar]3d64s2 |
| Cobalt (Co) | 27 | [Ar]3d74s2 |
| Nickel (Ni) | 28 | [Ar]3d84s2 |
| Copper (Cu) | 29 | [Ar]3d104s1 |
| Zinc (Zn) | 30 | [Ar]3d104s2 |
The number of electrons are increasing, but in a particular way. We know that as the number of electrons increases going across a period, there is more pull of these electrons toward the nucleus. However, with the d−electrons, there is some added electron-electron repulsion. Take a look at Table 10.5 and note the unusual electron configuration of chromium.
In chromium, there is a promotion of one of the 4s electrons to half fill the 3d sublevel, the electron-electron repulsions are less and the atomic size is smaller. The opposite holds true for the latter part of the row. Table 10.6 shows the first row of the transition metals along with their size.
| Element | Atomic Radii |
|---|---|
| Scandium (Sc) | 164 pm |
| Titanium (Ti) | 147 pm |
| Vanadium (V) | 135 pm |
| Chromium (Cr) | 129 pm |
| Manganese (Mn) | 137 pm |
| Iron (Fe) | 126 pm |
| Cobalt (Co) | 125 pm |
| Nickel (Ni) | 125 pm |
| Copper (Cu) | 128 pm |
| Zinc (Zn) | 137 pm |
We can see the trend in the 3d transition metals isn't quite as systematic as with the main group elements.
Lesson Summary
[edit | edit source]- Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located.
- Atomic size is difficult to measure because it has no definite boundary. The electrons surrounding the nucleus exist in an electron cloud. You can predict the probability of where the electrons are but not their exact location.
- Atomic size is determined indirectly.
- Atomic radius is a more definite and measureable way of defining atomic size. It is the distance from the center of one atom to the center of another atom in a homonuclear diatomic molecule.
- Rutherford led the way to determining the size of the atom with his gold foil experiment.
- There are three factors that help in the prediction of the trends in the Periodic Table: number of protons in the nucleus, number of shells, and shielding effect.
- The atomic size increases from the top to the bottom in any group as a result of increases in all of the three factors. (As the number of energy levels increases, the size must increase.)
- Going across a period (from left to right), the number of protons increases and therefore the nuclear charge increases. (Going across a period, the number of electron energy levels remains the same but the number of electrons increases within these energy levels. Therefore the electrons are pulled in closer to the nucleus.)
- Shielding is relatively constant since the core electrons remain the same.
- The trend in the periodic table is that as you move across the Periodic Table from left to right, the atomic radii decrease. This trend is not as systematic for the transition metals because other factors come into play.
Review Questions
[edit | edit source]- Why is the atomic size considered to have "no definite boundary"?
- How is atomic size measured?
- (a) using a spectrophotomer
- (b) using a tiny ruler (called a nano ruler)
- (c) indirectly
- (d) directly
- Draw a visual representation of the atomic radii of an iodine molecule.
- Which of the following would be smaller?
- (a) In or Ga
- (b) K or Cs
- (c) Te or Po
- Explain in your own words why Iodine is larger than Bromine.
- What three factors determine the trend of atomic size going down a group?
- What groups tend to show this trend?
- Which of the following would have the largest atomic radii?
- (a) Si
- (b) C
- (c) Sn
- (d) Pb
- Which of the following would have the smallest atomic radius?
- (a) 2s2
- (b) 4s24p3
- (c) 2s22p4
- (d) 4s2
- Arrange the following in order of increasing atomic radii: Tl, B, Ga, Al, In.
- Arrange the following in order of increasing atomic radii: Ge, Sn, C.
- Which of the following would be larger?
- (a) Rb or Sn
- (b) Ca or As
- Place the following in order of increasing atomic radii: Mg, Cl, S, Na.
- Describe the atomic size trend for the rows in the Periodic Table.
- Draw a visual representation of the periodic table describing the trend of atomic size.
- Which of the following would have the largest atomic radii?
- (a) Sr
- (b) Sn
- (c) Rb
- (d) In
- Which of the following would have the smallest atomic radii?
- (a) K
- (b) Kr
- (c) Ga
- (d) Ge
- Place the following elements in order of increasing atomic radii: In, Ca, Mg, Sb, Xe.
- Place the following elements in order of decreasing atomic radii: Al, Ge, Sr, Bi, Cs.
- Knowing the trend for the rows, what would you predict to be the effect on the atomic radius if an atom were to gain an electron? Use an example in your explanation.
- Knowing the trend for the rows, what would you predict to be the effect on the atomic radius if the atom were to lose an electron? Use an example in your explanation.
Vocabulary
[edit | edit source]- atomic radius
- One-half the distance between the centers of the two atoms of a homonuclear molecule.
- atomic size
- Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located.
- electron-electron repulsion
- The separation that occurs because electrons have the same charge.
- nuclear charge
- The number of protons in the nucleus.
- shielding effect
- The core electrons in an atom interfere with the attraction of the nucleus for the outermost electrons.
This material was adapted from the original CK-12 book that can be found here. This work is licensed under the Creative Commons Attribution-Share Alike 3.0 United States License
