Biomedical Engineering Theory And Practice/Neuro engineering
See also Wikipedia, Neural Engineering.
Neuroengineering is a discipline within biomedical engineering that uses engineering techniques to understand, repair, replace, or enhance neural systems.
Overview and History of Neuroengineering
[edit | edit source]Definition and Basic Principle
[edit | edit source]Neural Engineering is the highly interdisciplinary field of neuroscience, electrical engineering, clinical neurology, materials science, nanotechnology computer engineering and so on. Prominent goals in the field is to better understand and to mimic the functioning and dysfunctioning of the nervous system and to engineer appropriate augmentation and/or substitution for dysfunctioning parts of the nervous system.
Neural Engineering combines a broad range of engineering and basic science principles together with an wide range of biological and medical sciences. It connects basic and applied engineering and science R&D with basic and applied neuroscience.
Background and History
[edit | edit source]Electricity (in the form of electric fish) was used by ancient Egyptians and Romans for therapeutic purposes. During the 1790s, Italian physician Luigi Galvani demonstrated the electrical basis of nerve impulses when he made frog muscles twitch by jolting them with a spark from an electrostatic machine.[1]:67–71 Scientific fellows generally accepted Galvani’s views; but Alessandro Volta demonstrated that the electricity did not come from the animal tissue but was by the contact of different metals, brass and iron, in a moist environment. On the other hand, in another experiment, Galvani caused muscular contraction by touching the exposed muscle of one frog with the nerve of another. And he proved for the first time that bioelectric forces exist within living tissue. Guillaume Duchenne (September 17, 1806, in Boulogne-sur-Mer – September 15, 1875, in Paris) revived Galvani's research. He was the first to describe several nervous and muscular disorders and, in advancing medical treatment for them, created electrodiagnosis and electrotherapy.
In the mid-twentieth century, electrical recordings became popular as a window into neuronal function
Electrical Stimulation of Central Nervous System
[edit | edit source]
The different distribution of charges between the interior and the exterior of plasma membrane produce membrane potential (also transmembrane potential or membrane voltage). Two forces that establish and maintain resting membrane potential are passive (diffusion via channels) and active (sodium-potassium pump)[2]. With respect to the cytoplasmic side of neuron's membrane, typical values of the resting potential range from –70 to –80 millivolts. Like muscles, neurons use changes in their membrane potential as communication signals to receive, integrate, and send information. The difference in membrane potential can be produced by the permeability of the ions and the ion concentration on the two sides of the membrane. The opening and closing of ion channels can evoke a departure from the resting potential. This is called a hyperpolarization if the interior voltage becomes more negative (say from –70 mV to –80 mV) or depolarization if the interior voltage becomes less negative (say from –70 mV to –60 mV). In excitable cells, a clearly large depolarization can cause an action potential (long distance signals). But, the ion concentrations do not normally change very quickly. Therefore, graded potentials result from the passive electrical property of the neuronal membrane.
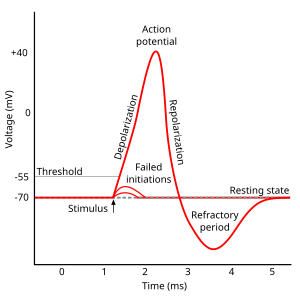
Action Potential
[edit | edit source]There are cell-to-cell communication with excitable membranes. They do not decrease in strength with distance and are referred to as "nerve impulse" or "spikes". The temporal sequence of action potentials generated by a neuron is called its "spike train". Stimulus changes permeability of neuron's membrane by opening specific voltage-regulated gated channels located on axons. Axons only could generate action potentials.
Generation of Action Potential (induced by depolarization) follows three sequential changes:
1.Sodium permeability increases and the membrane potential is on the contrary.
2.Sodium permeability decreases.
3.Potassium permeability increases and repolarization happens.
Graded Potential
[edit | edit source]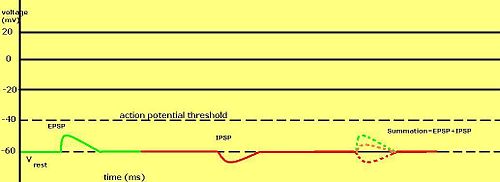
Voltage impulses in neuronal dendrites with various strength. Stimulation (by another neuron or as a special receptor) of the dendrites in a neuron produce a graded potential. Stimulation can occur in various ways like chemical stimulation (neurotransmitters, etc.), mechanical stimulation (certain pain receptors, hair receptor, etc.), light stimulation (photoreceptors) and a few others. Anyways, these stimulation bring the same result. Certain receptor protein ion channels on the dendrites are activated, and opened. This causes an influx (or efflux) of ions. It can cause either a depolarization (become less negative,-70 to -60mV,an excitatory response that may lead to an action potential) or hyperpolarization (become more negative, -70 to -80mV, an inhibitory response which makes it harder for an action potential to occur) according to the ion let in (or out). The more receptor protein ion channels that are stimulated the stronger, or more intense the signal. A slight stimulation of a receptor protein ion channel will often open the channel and accept ions.
However, a depolarization of threshold strength to reach the axon hillock, it generally needs multiple depolarizing receptor protein ion channels to open. Therefore, graded potentials are accumulated. The total polarizing effect of the ion channels adds together.One channel cannot stimulate an action potential, It takes multiple channels working together to depolarize a membrane enough to cause and action potential. Graded potentials (or receptor potentials) are short lived depolarizations or hyperpolarizations of an area of membrane. These changes cause local flows of current that decrease with distance.
The graded potential is proportional to a direct reflection of the intensity or strength of the stimulus. The more intense the stimulus, the more ion channels that are opened, and the greater the voltage change (hyper or de- polarization) and the farther the current flows.
Stimulated polarization happens as ions rush in. These ions accumulate near to the stimulated area. From there the surplus of ions radiates out in all directions, polarizing adjoining membranes. As this polarization spreads like a wave it leaves behind it a wake of formerly polarized membrane that very quickly returns to resting membrane potential.
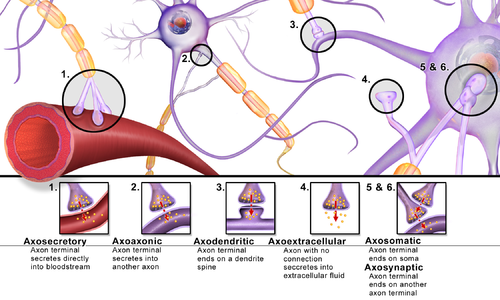
Transmission at Synapses
[edit | edit source]Like wires in our home's electrical system, nerve cells are connected to one another in circuits called neural pathways. Unlike wires in our home, nerve cells do not touch, but come close together at synapses. At the synapse, the two nerve cells are separated by synaptic cleft. The sending neuron is called the presynaptic cell, while the receiving one is called the postsynaptic cell. Nerve cells send chemical messages with neurotransmitters in a one-way direction through the synapse from presynaptic cell to postsynaptic cell.
The transmission process at synapses that uses the neurotransmitter is follows:
- Synthesis and storage of neurotransmitter
- Neurotransmitter Release
- Neurotransmitter Postsynaptic Receptors
- Inactivation of Neurotransmitters
Synthesis and storage of neurotransmitter
[edit | edit source]Neurotransmitter synthesized in neurons are divided into two major categories: small-molecule neurotransmitter and neuropeptide. Small-molecule neurotransmitters are synthesized within the axon terminal(e.g.acetylcholine (ACh)). Some of the precursors for the synthesis of these molecules are taken up by selective transporters on the membrane of the terminal. Others are byproducts of cellular processes within the neuron and are thus readily available. Neuropeptide are different from small-molecule neurotransmitters in both size and in the way of the synthesis. Neuropeptides generally range from 3 to 36 amino acids in length. So, they are larger than small-molecule neurotransmitters. Small neurotansmitters are made within the axon terminal as they need the simple enzymatic reactions but neuropeptides are made in the cell body as their synthesis requires peptide bond formation.The synthesis of a neuropeptide is similar to the process of any secretory protein within the cell. First, within the cell nucleus, during which a specific peptide-coding sequence of DNA,gene transcription takes place and It is used as a template to build a corresponding strand of messenger RNA. The mRNA then travels to a ribosome, where the process of translation begins. During translation, the sequence of the mRNA act as a code to string together a corresponding sequence of amino acids that will become the neuropeptide needed at the terminal. Before this molecule can be transported to the terminal for release into the synaptic cleft, it must be processed in the endoplasmic reticulum (ER), packaged in the golgi apparatus, and transported in storage vesicles down the axon to the terminal.
Once they are synthesized, neurotransmitters, both small molecules and neuropeptides, are stored in vesicles within the axon terminal until an action potential arrives and they are released. Most small-molecule neurotransmitters are stored in small vesicles in the range of 40~60 nm in diameter.The vesicles that store neuropeptides are larger, ranging from 90 to 250 nm in diameter.
Neurotransmitter Release
[edit | edit source]Neurotransmitter-including vesicles are stored at the terminal of the neuron in active zone or near active zone. These vesicles are held in place by Ca2+-sensitive vesicle membrane proteins binding to actin filaments, microtubules, and various components of the cytoskeleton. When an action potential reaches the terminal of a presynaptic neuron, voltage-dependent calcium (Ca2+) channels in the pre-synaptic membrane open and Ca2+ rushes in. This influx of calcium ions triggers a series of events, which eventually release the neurotransmitter from a storage vesicle into the synaptic cleft.
Neurotransmitter Postsynaptic Receptors
[edit | edit source]After release into the synaptic cleft, neurotransmitters communicate with receptor proteins on the membrane of the postsynaptic cell, causing ionic channels on the membrane to open or close. When these channels open, depolarization occurs, involving the initiation of another action potential. There are two types of postsynaptic receptors that recognize neurotransmitters: Ionotropic receptors called ligand-gated ion channels and metabotropic receptors called G-protein linked receptors.
Inactivation of Neurotransmitters
[edit | edit source]After the recognition of a neurotransmitter molecule by a post-synaptic receptor, it is released back into the synaptic cleft. Once in the synapse, it must be chemically inactivated or quickly removed in order to avoid constant stimulation of the post-synaptic cell and an excessive firing of action potentials.
Neurotransmitter metabolism
[edit | edit source]-
Acetylcholine metabolisme
-
Catecholamine and trace amine biosynthesis
-
GABAergic-synapse
Neurotransmitter
[edit | edit source]Neurotransmitters are the brain chemicals that communicate information throughout our brain and body. Communication of information between neurons is done by movement of neurotransmitter across a small gap called the synapse. Neurotransmitters are released from one neuron at the presynaptic nerve terminal. Neurotransmitter agents include agonists, antagonists, degradation inhibitors, uptake inhibitors, depleters, precursors, and modulators of receptor function[3]. Neurotransmitters can be divided into amino acids, peptides, and monoamines.
- Small Molecule Neurotransmitter Substances:Acetylcholine (ACh), Dopamine (DA), Norepinephrine (NE), Serotonin (5-HT), Histamine, Epinephrine, Amino Acids, Gamma-aminobutyric acid (GABA), Glycine, Glutamate, Aspartate
- Neuroactive Peptides(partial list):bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, enkephalin,dynorphin,insulin,gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, angiotensin II
- sleep peptides: galanin,thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide
- Soluble Gases:Nitric Oxide (NO), Carbon Monoxide
Table: Selected neurotransmitter and function
How it works
[edit | edit source]Neuromodulation and Neuroaugmentation
[edit | edit source]Neuromodulation is defined as “the alteration of nerve activity through the delivery of electrical stimulation or chemical agents to targeted sites of the body,”by the International Neuromodulation Society. It is carried out to normalize – or modulate – nerve function. Neuromodulation is the process in which diverse neurotransmitters in the nervous system control various populations of neurons (one neuron uses different neurotransmitters to connect to several neurons). Neuromodulation can involve electromagnetic stimuli such as a strong magnetic field repetitive transcranial magnetic stimulation, a very small electric current or, potentially, light optogenetics.[4]Neuromodulation use medical device technologies to enhance or suppress activity of the nervous system for the treatment of disease. These technologies contain implantable as well as non-implantable devices that deliver electrical, chemical or other agents to reversibly modify brain and nerve cell activity.[4]
Neuromuscular Electrical Stimulation(NMES)
[edit | edit source]Neuromuscular electrical stimulation can be widely categorized as functional or therapeutic. Therapeutic neuromuscular stimulation refers to the use of stimulation of paralyzed muscles to minimize specific impairments like motor weakness, spasticity, limited range of motion and cardiovascular deconditioning. Functional neuromuscular stimulation(FNS) refers to the use of stimulation to activate paralyzed muscles at an exact sequence to assist in the performance of daily lives(ADLs) or to provide stability to a joint to maintain original biomechanical property and the function. Devices or systems that provide FNS are called as neuroprosthetics.[5]
Neuroregeneration
[edit | edit source]
Neuroregeneration indicates to the regrowth or repair of nervous tissues, cells or cell products[6]. The processes that occur in regeneration can be divided into the following major events: Wallerian degeneration, axon regeneration/growth, and nerve reinnervation[7]. In other views, neuroregneration contains neurogenesis, neuroplasticity, and neurorestoration--implantation of viable cells as a therapeutical approach[8]. Neuroregeneration mechanisms are very different between the peripheral nervous system (PNS) and the central nervous system (CNS) in view of the extents and the speeds.
Peripheral nervous system regeneration
[edit | edit source]Peripheral nerve damage is classified in the Seddon classification based on the extent of damage to the nerve and the surrounding connective tissue[9].The lowest degree of nerve injury with a temporary interruption of conduction without loss of axonal continuity is called neurapraxia[10]. The second degree called axonotmesis is related to the loss of the relative continuity of the axon and its covering of myelin, but it preserves the connective tissue framework of the nerve ( the encapsulating tissue, the epineurium and perineurium, are preserved ).[11]. The last degree called neurotmesis is a total severance or disruption of the entire nerve fiber[12].
Unlike in the central nervous system, regeneration in the peripheral nervous system is possible[13]. Wallerian degeneration occurs before nerve regeneration. During Wallerian degeneration Schwann cells and macrophages interact to remove specially myelin and the damaged axon, from the distal injury site. Bands of Büngner are formed when un-innervated Schwann cells proliferate and the remaining connective tissue basement membrane forms endoneurial tubes. Bands of Büngner are important in order to guide the regrowing axon[14].
At the neuronal cell body, a chromatolysis occurs in which the nucleus moves to the periphery of the cell body and the endoplasmic reticulum breaks up and disperses. Nerve damage change the metabolic function of the cell. The cell tries to produce molecules for growth and repair instead of producing molecules for synaptic transmission. These factors contains GAP-43, tubulin and actin. When the cell is ready for axon regeneration, chromatolysis is reversed[15].
Axon regeneration is characterized by the formation of a growth cone[16]. The growth cones are located on the very tips of nerve cells on structures called axons and dendrites. They include bundles of actin filaments (F-actin) that give them shape and support. The growth cone interacts with molecules produced by Schwann cells such as laminin and fibronectin[14].
Central nervous system regeneration
[edit | edit source]Unlike peripheral nervous system regeneration, that of the central nervous system is not followed by extensive regeneration. It is limited by the inhibitory influences of the glial and extracellular environment. Regeneration in the central nervous system (CNS) would be possible that new neurons, generated from proliferation of endogenous stem/progenitor cells or under administration of exogenous stem/precursor cells with potential to substitute for lost tissue, will differentiate, survive, and integrate into existing neural networks and that axons regenerate[17]. Since several studies have reported about the existence of adult neural stem cells[18][19][20], the concepts of neuroplasticity and neural stem cells based on neural tissue engineering led to the idea of neurorestoration as an substutive therapy for neurodegenerative disorders in CNS[17] [21] .
Research and Application
[edit | edit source]Neural Imaging
[edit | edit source]Neuroimaging contains a variety of techniques to directly or indirectly image of the structure, function/pharmacology of thenervous system. It is a relatively new fields within medicine, neuroscience and psychology.[22] Physicians who specialize in the performance and interpretation of neuroimaging in the clinical setting are neuroradiologists.
Neuroimaging is classified into two categories:
- Structural imaging, which deals with the structure of the nervous system and the diagnosis of gross (large scale) intracranial disease (such as tumor), and injury.
- functional imaging for diagnosing metabolic diseases and lesions on a finer scale (such as Alzheimer's disease) and for neurological and cognitive psychology research and building brain-computer interfaces.
Neural Networks
[edit | edit source]See also Artificial neural network
A Neural Networks(An Artificial Neural Network:ANN) is an information processing paradigm that is inspired by animals central nervous systems, such as the brain, process information. The key element of this paradigm is the novel structure of the information processing system. It is composed of a large number of highly interconnected neurons which can compute values from inputs. The first artificial neuron was developed in 1943 by the neurophysiologist Warren McCulloch and the logician Walter Pits[23]. Many important studies have been boosted by cheap computer emulations. Neural network studies slow down after publishing machine learning research by Marvin Minsky and Seymour Papert[24] (1969). They found out two key issues with the computational machines that processed neural networks. The first issue was that single-layer neural networks could not process the exclusive-or circuit. The second issue was that computers were not sophisticated enough to effectively handle the long run time required by large neural networks. Neural network research slowed until computers got greater processing power. Also key later advances was the backpropagation algorithm which effectively solved the exclusive-or problem (Werbos 1975).[25]
Artificial Neurons
[edit | edit source]
An artificial neuron is a mathematical function as a model of biological neurons. Artificial neurons are the constitutive units in an artificial neural network. For a given artificial neuron, let suppose that there are m + 1 inputs with signals x0 through xm and weights w0 through wm. Usually, the x0 input would be the value +1, which makes it a bias input with wk0 = bk. This leaves only m actual inputs to the neuron: from x1 to xm.
The output of kth neuron is:
Where (phi) is the transfer function[26]. The output is similar to the axon of a biological neuron, and its value propagates to input of the next layer, through a synapse. It may also exit the system, as part of an output vector. Its transfer function weights are calculated and threshold value are predetermined. The limitation is simple artificial neurons, such as the McCulloch–Pitts model, are sometimes expressed as "caricature models", since they try to reflect one or more neurophysiological observations, but without regard to realism.[27]
Neural Interface
[edit | edit source]Neural interface system provides a direct communication pathway between the nervous system and the outside world by stimulating or by recording from neural tissue to help people with sensory, motor, or other disabilities of neural function. These research is called a new branch of experimental neuroscience, variously named brain-machine interfaces (BMIs), brain-computer interfaces (BCIs), neural prostheses, or neural interface systems (NISs). An electronics package in each device activates an array of tiny electrodes that contact healthy neurons in the body. Signals by the electrodes bypass damaged areas of the brain or part of the nervous system to restore function, block pain, or prevent seizures. Although electrical stimulation systems have already broadly used for clinical application, neural interfaces that record and decipher neural signals are just starting for clinically systems to assist impaired people. The examples of the successful neural interfaces include the cochlear implant[28][29] to provides a sense of sound to people with severe hearing impairment and the deep brain stimulator (DBS) [30] to help prevent seizures in patients with epilepsy and Parkinson’s disease.
Since the late 1990, neural interface studies have developed remarkably on the basis of the closed-loop control using neuronal spikes. Chapin and colleagues showed a rat's ability to control a one-dimensional feeder through multielectrode recordings from sensorimotor cortex[31]. After that, a lot of studies(Carmena et al. 2003[32], Musallam et al. 2004[33], Santhanam et al. 2006[34],Taylor et al. 2002[35] and so on) demonstrated that closed-loop control in primates is available. The common closed-loop neural interface system is composed of four components: (1) a recording array that extracts neural signals, (2) a decoding algorithm that translates these neural signals into a set of command signals, (3)an output device that is controlled by these command signals, and (4) sensory feedback in the form of vision and potentially other sensory modalities.
Neural implants must be designed to be as tiny as possible so as to be minimally invasive, in special areas srrounding the brain, eyes or cochlea. These implants communicate with their prosthetic counterparts wirelessly. In addition, power is currently received through wireless power transmission through the skin. Usually, the tissue near the implant is highly sensitive to temperature rise. It means that power consumption must be minimal to avoid tissue damage.[36]

Input Electrode
[edit | edit source]

Intracranial electrodes is composed of conductive electrode arrays implanted on a polymer or silicon, or a wire electrode with an exposed tip and insulation for the part that stimulation or recording is not needed.
Current implantable microelectrodes could not record single- or multi-unit activity depending on a chronic scale. Lebedev and Nicolelis reviewed the specific needs in the field to truly improve the technology to the level of clinical implementation. In short, the 4 requirements in their review are:
- Consistent long term (over the years) recording of large neuronal populations in multiple brain areas;
- Efficient processing of recorded data;
- Incorporation of feedback into the user’s body using native plasticity;
- Advances in prosthetic technology to create artificial limbs which can reproduce the full motion.[37][38]
Hardware
[edit | edit source]Chips have incorporated a various amount of data compression on chip[39], containing spike sorting (Chae et al., 2009). While a lot of systems have too high power consumption in order to be powered from a battery implant, Sarpeshkar et al have fabricated amplifiers and analog to digital converters at a power costless than 9 uW per 20 ksps channel[40]. Although the small area and low power consumption of available integrated circuits is enough to process most of channels of neural data, some problem should be resolved. For instance, how long the implant can stay in the body? Electronics coated with 6 um parylene C have been proved to work for up to 276 days[41]. Commercial systems such as Plexon, Tucker Davis, NeuroLynx, and so on depend on hermetically sealed feed-through connectors into a welded titanium casing, in which the electronics are housed. But, Brain machine interfaces which use hundreds of electrodes and high-density miniature hermetic feedthroughs do not exist yet.
Decoder process
[edit | edit source]Performance Evaluation
[edit | edit source]Neurorobotics
[edit | edit source]Neurorobotics is the part of neuroscience with robotics, dealing with the study and application of science and technology of embodied autonomous neural systems like brain-inspired algorithms. Neurorobotics starts from the idea "the brain is embodied and the body is embedded in the environment." A simulated environment can provide unintentional biases to the model. In addition, real environments are unpredictable, multimodal, and noisy; an artificial design of such an environment would be difficult to simulate. Therefore, most neurorobots need to work with the real world, instead of a simulated environment.[42]

There are a lot of classes of neurobiologically inspired robotic devices. The three common types of neurorobots are used to study motor control, memory, and action selection.
- Motor control and locomotion
- Learning and memory systems
- Value systems and action selection
Motor control and locomotion
[edit | edit source]Neurorobots have proved good for studying animal locomotion and motor control, and for developing robot controllers. Locomotion control in robots is designed by a number of neurologically inspired theories based on the action of motor systems. Neural models of central pattern generators, clumps of motorneurons capable of driving a repetitive behavior, have been used for the vertebrate-legged locomotion like four-legged walking robots [43] [44]. Another method for motor control is to use a predictive controller to convert awkward, error prone movements into smooth, accurate movements. Using these ideas, the robot designed to avoid obstacles [45][46], produce accurate eye [47] and generate adaptive arm movements [48][49][50].
Learning and memory systems
[edit | edit source]Robots designed to test theories of animal memory systems. Currently, many studies focus on the memory system of rats, the rat hippocampus, dealing with place cells, which fire specifically at a spatial location that has been learned.[51][52]
Value systems and action selection
[edit | edit source]Action selection studies deal with negative or positive feedback to an action and its outcome. Examples of this studies in the brain contain the dopaminergic, cholinergic, and noradrenergic systems as neurotransmitters such as dopamine or acetylcholine positively reinforce neural signals that are beneficial[53][54][55]. One study of such interaction involved the robot Darwin VII, which used visual, auditory, and a simulated taste input to "eat" conductive metal blocks. The randomly chosen good blocks had a striped pattern on them while the bad blocks had a circular shape on them. The taste sense was simulated by conductivity of the blocks. Doya’s group has been studying the effect of multiple neuromodulators in the “Cyber-rodent” such as two-wheeled robots that move autonomously in an environment [56]. These robots could move for self-preservation and self-reproduction exemplified by searching for and recharging from battery packs on the floor and then communicating this information to other robots through their infrared communication ports. Including examining how neuromodulators such as dopamine can influence decision making, neuroroboticists have been investigating the basal ganglia as a model that mediates action selection [57]. Prescott and colleagues embedded a model of the basal ganglia in a robot that had to select from several actions relying on the environment.
Neural tissue regeneration
[edit | edit source]Surgical connection in PNS
[edit | edit source]In the peripheral nervous system, nerves can regenerate on their own if injuries are small. However, In the case of a small gap like between the proximal and distal nerve ends, it is possible to surgically reconnect the severed nerve by taking the two ends of the nerve and suturing them together. When suturing the nerves together, the fascicles of the nerve are each reconnected, bridging the nerve together. This method does not work over gaps of longer distances because of the tension that should stay on the nerve endings. This tension interrupt the nerve regeneration.[58]
Tissue grafts in PNS
[edit | edit source]Tissue grafts use nerves or other materials to bridge the two ends of the severed nerve. Tissue grafts are categorized into autologous tissue grafts, nonautologous tissue grafts, and acellular grafts. Autologous tissue grafts means the transfer of tissue from one site to another on the same body[59]. These autologous nerve grafts are the current standard for PNS nerve grafting as it is highly biocompatible, but there are some problem related to harvesting the nerve from the patients themselves and storing a large amount of autologous grafts for future use. nonautologous tissue grafts and acellular grafts (containing ECM-based materials) are tissues that do not come from the patient, but can be harvested from cadavers or animals. As they are not from the patients, it is easy to get it but these tissue have some difficulty in the potential of disease transmission. Currently, there are on being investigated to increase the efficacy of nonautologous tissue grafts.[58]
Nerve guidance channel
[edit | edit source]
A nerve guidance conduit as opposed to an autograft is an artificial means of guiding axonal regrowth to help nerve regeneration and is one of clinical treatments for nerve injuries. Because of the limited availability of donor tissue and functional recovery in autografting, neural tissue engineering studies has focused on the bioartificial nerve guidance conduits as an alternative treatment. Similar techniques are also being studied for nerve repair in the spinal cord but nerve regeneration in the central nervous system would be challenged because its axons do not regenerate in their native environment.[60] Guidance methods reduce scarring of the nerves, increasing the functionality of the nerves to transmit action potentials after reconnection. In this method, two types of materials are used:natural materials and synthetic materials.
Biological materials mostly have good biocompatibility and can be easily degraded as it is from nature. However, unfortunately, it has the limitation in order to control mechanical properties and degradation rates. Besides, there is always the possibility that naturally-derived materials may cause an immune response or contain microbes[61]. In the production of naturally-derived materials there will be unexpected results in large-scale purification process.[62] Some other problems is plaguing natural polymers could not support growth across long lesion gaps due to the possibility of collapse, scar formation, and early re-absorption.[62] Biological materials that have the potential to promote nerve repair are polysialic acid (PSA),collagen,spider silk fiber,silkworm silk fibroin, chitosan, aragonite, alginate, hyaluronic acid, glycosaminoglycans, Laminin, chitosan and so on.
Synthetic materials also provide an another method for tissue regeneration. synthetic polymers may be non-degradable or degradable. But, for neural tissue engineering degradable materials are preferred as long-term effects such as inflammation and scar could damage nerve function. Mechanical properties and degradation rates of the polymers can be controlled according to the purpose, and they eliminate the concern for immunogenicity.[61] There are many different synthetic materials currently being used in neural tissue engineering. However, the problem is a lack of biocompatibility and bioactivity of these materials. It means these polymers are not perfect for promoting cell attachment, proliferation, and differentiation.[63] Currently, the materials most commonly researched mainly focus on biodegradable polyesters such as copolymer or blend of poly lactic acid, poly glycolic acid, polycaprolactone, polyethyleneglycol(FDA approved), biodegradable polyurethane, other polymers, and biodegradable glass are also being investigated. Other potentials for synthetic materials are conducting polymers and biologically modified polymers to promote cell axon growth and maintain the axon channel.[58]
Implantation of stem cells or neural tissue
[edit | edit source]
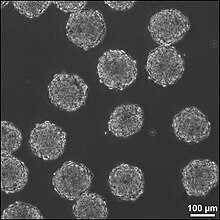
It is possible to artificially create tissue outside of the body to implant into the injury site. This method could treat injuries with large cavities, where a lot of neural tissue needs to be replaced and regenerated. Neural tissue could be grown in vitro with neural stem or progenitor cells in a 3D scaffold, forming embryoid bodies (EBs). These EBs is composed of a sphere of stem cells, where the inner cells are undifferentiated neural cells, and the surrounding cells are more differentiated. 3D scaffolds is for transplant of tissue to the injury site and building the suitable interface between the artificial and the brain tissue. The scaffolds should be biodegradable and biocompatible. It need to fit injury site, close to existing tissue and support growing cells and tissues. The combination of using the stem cells and scaffolds increase the survival of the stem cells in the injury site, increasing the efficacy of the treatment.[64]
Table. Preparation of scaffold
| Preparation of scaffold | Characteristics |
|---|---|
| CAD/CAM Technologies | Example |
| Electrospinning | Example |
| Emulsification/Freeze-drying | Example |
| Gas Foaming | Example |
| Nanofiber Self-Assembly | Example |
| Textile technologies | Example |
| Thermally Induced Phase Separation (TIPS) | Example |
| Nanofiber Self-Assembly | Example |
Delivery of molecules
[edit | edit source]
Molecules that enhance the regeneration of neural tissue, including pharmaceutical drugs, growth factors known as morphogens, and miRNA can also be directly injected to the injury site of the damaged CNS tissue. The strategies for brain drug delivery may be widely categorized into invasive (neurosurgical-based), pharmacologic-based, or physiologic-based. The neurosurgical-based strategies contain intraventricular drug infusion, intracerebral implants, and BBB disruption. The pharmacologic-based strategies include the use of particles such as microspheres, nanospheres made by emulsion, spraydrying, dispersion and so on. [65][66]
Advanced techniques
[edit | edit source]Neural enhancement
[edit | edit source]
Neural enhancement or human enhancement can be used for treating illness and disability, but also for enhancing human characteristics and capacities.[68] In some circles, the "human enhancement technologies" is synonymous with emerging technologies or converging technologies or transhumanism.[69] In other views, "Human enhancement" is roughly synonymous with human genetic engineering,[70][71] it refers usually to the general application of the combination of nanotechnology, biotechnology, information technology and cognitive science(NBIC) to improve human lives.[69] Deep brain stimulation has already proved to provide therapeutic benefits for the patients currently using this treatment for Parkinson's disease, essential tremor, dystonia, chronic pain, major depression and OCD[72]. Ethical issues with human enhancement would be that neural engineers need to grapple with as they develop techniques. Some controversial idea is that human enhancement maintain or modify their own minds and bodies[73]. In addition, there are fear that some enhancements will create unfair physical or mental advantages to those who can and will use them and will increase the gap between the "haves" and "have-nots"[74][75][76][77]. However,some advocates advance "human enhancement technologies" and prefer the term "enablement" to "enhancement"[78]. And they try to defend and develop independent safety testing of the technologies and affordable, universal access to these technologies.[79]
Deep brain stimulation
[edit | edit source]
Deep brain stimulation (DBS) is a neurosurgical procedure which implant a tiny medical device called a brain pacemaker, which sends electrical impulses through implanted electrodes to specific area of the brain for the treatment of movement and affective disorders.The Food and Drug Administration (FDA) approved DBS as a treatment for Parkinson's disease in 2002,[80] dystonia in 2003,[81] and obsessive-compulsive disorder (OCD) in 2009.[82] DBS has been also used to treat diverse affective disorders, including major depression but these applications of DBS have yet been FDA-approved.
The deep brain stimulation system is composed of three components: the implanted pulse generator (IPG), the lead, and the extension. The IPG is a battery -powered neurostimulator enclosed in a titanium housing, which sends electrical pulses to the brain to interfere with neural activity at the target site. The lead is a coiled wire insulated in biocompatible but nondegradable polymer like polyurethane with four platinum or iridium electrodes and is placed in one or two different nuclei of the brain. The lead is connected to the IPG by the extension. It is an insulated wire that runs below the skin, from the head, down the side of the neck, behind the ear to the IPG, which is placed below the clavicle or, in some cases, the abdomen.[83]The leads are placed in the brain according to the type of symptoms to be addressed. The IPG can be calibrated by a neurologist,nurse, or trained technician.[84]
All three components are surgically implanted in the brain. Lead implantation may cause local anesthesia or general anesthesia ("asleep DBS"). A hole about 14 mm in diameter is drilled in the skull and the probe electrode is inserted. During the awake procedure with local anesthesia, feedback from the patient determines the best placement of the permanent electrode. During the asleep procedure, intraoperative MRI guidance is used for the determination of the electrode. [85] Generally, the IPG and extension leads are installed under general anesthesia.[86]
Further Reading
[edit | edit source]- Bronzino, Joseph D. (April 2006). The Biomedical Engineering Handbook, Third Edition. [CRC Press]. ISBN 978-0-8493-2124-5.
- Villafane, Carlos, CBET. (June 2009). Biomed: From the Student's Perspective, First Edition. [Techniciansfriend.com]. ISBN 978-1-61539-663-4.
{{cite book}}: CS1 maint: multiple names: authors list (link)
Practise
[edit | edit source]Reference
[edit | edit source]- ↑ Whittaker, E. T. (1951), A history of the theories of aether and electricity. Vol 1, Nelson, London
- ↑ Franco R, Bortner CD, Cidlowski JA (January 2006). "Potential roles of electrogenic ion transport and plasma membrane depolarization in apoptosis". J. Membr. Biol. 209 (1): 43–58. doi:10.1007/s00232-005-0837-5. PMID 16685600.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ National Library of Medicine - Medical Subject Headings,Neutrotransmitter
- ↑ a b "International Neuromodulation Society home page". Retrieved 1 October 2013.
- ↑ Joel A. DeLisa, Bruce M. Gans, Nicholas E. Walsh(2005),Physical Medicine and Rehabilitation: Principles and Practice,Lippincot Williams & Wilkins,p.1405
- ↑ Wikipedia, Neuroregeneration
- ↑ Trauma and Wallerian Degeneration
- ↑ Enciu AM et al.Neuroregeneration in neurodegenerative disorders,BMC Neurology 2011, 11:75
- ↑ "Peripheral Nerve Injuries".
- ↑ Otto D.Payton & Richard P.Di Fabio et al.Manual of physical therapy. Churchill Livingstone Inc. ISBN 0-443-08499-8
- ↑ "Classification of Nerve Injuries".
- ↑ Otto D.Payton & Richard P.Di Fabio et al.Manual of physical therapy. Churchill Livingstone Inc. Page:24. ISBN 0-443-08499-8
- ↑ Fenrich, Keith; Gordon, Tessa (2004). "Axonal Regeneration in the Peripheral and Central Nervous Systems - Current Issues and Advances". Canadian Journal of Neurological Sciences. 31 (2): 142–156. ISSN 0317-1671.
- ↑ a b Campbell, William W. (31 August 2008). "Evaluation and management of peripheral nerve injury". Clinical Neurophysiology. 119 (9): 1951–1965. doi:10.1016/j.clinph.2008.03.018. PMID 18482862.
- ↑ Burnett, Mark G.; Zager, Eric L. (2004). "Pathophysiology of Peripheral Nerve Injury: A Brief Review". Medscape Today: Neurosurgical Focus. ISSN 1092-0684. Retrieved 11 August 2013.
- ↑ Yoo, S (2010). "Dynamics of axonal mRNA transport and implications for peripheral nerve regeneration". Experimental Neurology. 1. 223: 19-27. doi:10.1016/j.expneurol.2009.08.011. PMC 2849851. Retrieved 1 April 2014.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ↑ a b Johansson BB: Regeneration and plasticity in the brain and spinal cord. J Cereb Blood Flow Metab 2007, 27:1417-1430
- ↑ McKay R: Stem cells in the central nervous system.Science 1997, 276:66-71.
- ↑ Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D: Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells.Neuron 1994, 13:1071-1082.
- ↑ Shihabuddin LS, Palmer TD, Gage FH: The search for neural progenitor cells: prospects for the therapy of neurodegenerative disease.Mol Med Today 1999, 5:474-480
- ↑ D. Kacy Cullen et al., Neural Tissue Engineering for Neuroregeneration and Biohybridized Interface Microsystems In vivo (Part 2), Critical Reviews™ in Biomedical Engineering, Volume 39, 2011 Issue 3, pages 241-259
- ↑ Filler, Aaron (12 July 2009). "The History, Development and Impact of Computed Imaging in Neurological Diagnosis and Neurosurgery: CT, MRI, and DTI". Nature Precedings. doi:10.1038/npre.2009.3267.4.
- ↑ McCulloch, Warren; Walter Pitts (1943). "A Logical Calculus of Ideas Immanent in Nervous Activity". Bulletin of Mathematical Biophysics. 5 (4): 115–133. doi:10.1007/BF02478259.
- ↑ Minsky, M.; S. Papert (1969). An Introduction to Computational Geometry. MIT Press. ISBN 0-262-63022-2.
- ↑ Werbos, P.J. (1975). Beyond Regression: New Tools for Prediction and Analysis in the Behavioral Sciences.
- ↑ wikipedia,artificial neuron
- ↑ F. C. Hoppensteadt and E. M. Izhikevich (1997). Weakly connected neural networks. Springer. p. 4. ISBN 978-0-387-94948-2.
- ↑ Gifford RH, Shallop JK, Peterson AM. Speech recognition materials and ceiling effects: considerations for cochlear implant programs. Audiol. Neurootol. 2008;13:193–205.
- ↑ Wilson BS, Dorman MF. Cochlear implants: a remarkable past and a brilliant future. Hear. Res. 2008;242:3–21.
- ↑ Arle JE, Alterman RL. Surgical options in Parkinson's disease. Med. Clin. North. Am. 1999;83:483–98. vii.
- ↑ Chapin JK, Moxon KA, Markowitz RS, Nicolelis MA. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat. Neurosci. 1999;2:664–70.
- ↑ Carmena JM, Lebedev MA, Crist RE, O'Doherty JE, Santucci DM, et al. Learning to control a brain-machine interface for reaching and grasping by primates. Public Library Sci. Biol. 2003;1:1–16.
- ↑ Musallam S, Corneil BD, Greger B, Scherberger H, Andersen RA. Cognitive control signals for neural prosthetics. Science. 2004;305:258–62.
- ↑ Santhanam G, Ryu SI, Yu BM, Afshar A, Shenoy KV. A high-performance brain-computer interface. Nature. 2006;442:195–98.
- ↑ Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296:1829–32.
- ↑ Daniel Garrison. "Minimizing Thermal Effects of In Vivo Body Sensors". Retrieved May 5, 2010.
- ↑ Lebedev, M.A., Brain–machine interfaces: past, present and future. Trends in Neuroscience, 2006. 29(9): p. 11.
- ↑ Nicolelis, M.A.L., Reconstructing the Engram: Simultaneous, Multisite, Many Single Neuron Recordings. Neuron, 1997. 18: p. 9.
- ↑ O'Driscoll, S.; Meng, T.H.; Shenoy, K.V. and Kemere, C. (2006). Neurons to Silicon: Implantable Prosthesis Processor IEEE ISSCC (talk) : 552-553.
- ↑ Sarpeshkar, R. et al. (2008). Low-Power Circuits for Brain–Machine Interfaces IEEE Trans BioCAS 2(3): 173-183
- ↑ Sharma, A. et al. (2011). Long term in vitro functional stability and recording longevity of fully integrated wireless neural interfaces based on the Utah Slant Electrode Array J. Neural Eng. 8: 1-7
- ↑ Chiel, H. J., & Beer, R. D. (1997). The brain has a body: adaptive behavior emerges from interactions of nervous system, body and environment. [Editorial Material]. Trends in Neurosciences, 20(12), 553-557.
- ↑ Ijspeert, A. J., Crespi, A., Ryczko, D., and Cabelguen, J. M. (2007). From swimming to walking with a salamander robot driven by a spinal cord model. Science 315, 1416-1420.
- ↑ Kimura, H., Fukuoka, Y., and Cohen, A. H. (2007). Biologically inspired adaptive walking of a quadruped robot. Philos Transact A Math Phys Eng Sci 365, 153-170
- ↑ McKinstry, J. L., Edelman, G. M., and Krichmar, J. L. (2006). A cerebellar model for predictive motor control tested in a brain-based device. Proc Natl Acad Sci U S A 103, 3387-3392
- ↑ Porr, B., and Worgotter, F. (2003). Isotropic sequence order learning. Neural Comput 15, 831-864
- ↑ Dean, P., Mayhew, J. E., Thacker, N., and Langdon, P. M. (1991). Saccade control in a simulated robot camera-head system: neural net architectures for efficient learning of inverse kinematics. Biol Cybern 66, 27-36
- ↑ Dean, P., Mayhew, J. E., Thacker, N., and Langdon, P. M. (1991). Saccade control in a simulated robot camera-head system: neural net architectures for efficient learning of inverse kinematics. Biol Cybern 66, 27-36
- ↑ Eskiizmirliler, S., Forestier, N., Tondu, B., and Darlot, C. (2002). A model of the cerebellar pathways applied to the control of a single-joint robot arm actuated by McKibben artificial muscles. Biol Cybern 86, 379-394
- ↑ Hofstotter, C., Mintz, M., and Verschure, P. F. (2002). The cerebellum in action: a simulation and robotics study. Eur J Neurosci 16, 1361-1376.
- ↑ O'Keefe, J., and Nadel, L. (1978). The hippocampus as a cognitive map (Oxford: Clarendon Press).
- ↑ Mataric, M. J. (1998). Behavior-based robotics as a tool for synthesis of artificial behavior and analysis of natural behavior. [Review]. Trends in Cognitive Sciences, 2(3), 82-87.
- ↑ Aston-Jones, G., and Bloom, F. E. (1981). Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1, 887-900
- ↑ Hasselmo, M. E., Hay, J., Ilyn, M., and Gorchetchnikov, A. (2002). Neuromodulation, theta rhythm and rat spatial navigation. Neural Netw 15, 689-707
- ↑ Schultz, W., Dayan, P., and Montague, P. R. (1997). A neural substrate of prediction and reward. Science 275, 1593-1599.
- ↑ Doya, K., and Uchibe, E. (2005). The Cyber Rodent Project: Exploration of Adaptive Mechanisms for Self-Preservation and Self-Reproduction. Adaptive Behavior 13, 149 - 160.
- ↑ Prescott, T. J., Montes Gonzalez, F. M., Gurney, K., Humphries, M. D., and Redgrave, P. (2006). A robot model of the basal ganglia: behavior and intrinsic processing. Neural Netw 19, 31-61
- ↑ a b c Schmidt, Christine; Jennie Leach (June 2003). "Neural Tissue Engineering: Strategies for Repair and Regeneration". Annuls Review of Biomedical Engineering. 5: 293–347.
- ↑ Free dictionary, autologous graft
- ↑ Schmidt, C. E.; Leach, J. B. (August 2003). "Neural tissue engineering: strategies for repair and regeneration". Annual Review of Biomedical Engineering. 5: 293–347. doi:10.1146/annurev.bioeng.5.011303.120731. PMID 14527315.
- ↑ a b Lavik, E.; Langer, R. (July 2004). "Tissue engineering: current state and perspectives". Applied Microbiology Biotechnology. 65 (1): 1–8. doi:10.1007/s00253-004-1580-z. PMID 15221227.
- ↑ a b Cai, J. (November 2005). "Permeable guidance channels containing microfilament scaffolds enhance axon growth and maturation". Journal of Biomedical Materials Research Part A. 75A (2): 374–386. doi:10.1002/jbm.a.30432. PMID 16088902.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ↑ Wu, Y. (2006). "Self-assembled IKVAV peptide nanofibers promote adherence of PC12 cells". Journal of Huazhong University of Science and Technology (Medical Science). 26 (5): 594–596. doi:10.1007/s11596-006-0530-7. PMID 17219978.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ↑ Forraz, N.; Wright, K. E.; Jurga, M.; McGuckin, C. P. (2013). "Experimental therapies for repair of the central nervous system: stem cells and tissue engineering.". Journal of Tissue Engineering and Regenerative Medicine 7: 523–536. doi:10.1002/term.552.
- ↑ William M Pardridge,Drug Delivery to the Brain,Journal of Cerebral Blood Flow & Metabolism (1997) 17, 713–731
- ↑ Menei P, Montero-Menei C, Venier MC, Benoit JP,Drug delivery into the brain using poly(lactide-co-glycolide) microspheres,Expert Opin Drug Deliv. 2005 Mar;2(2):363-76
- ↑ "''Converging Technologies''". Wtec.org. Retrieved 2012-05-18.
- ↑ Enhancement Technologies Group (1998). "Writings by group participants". Retrieved 2007-02-02.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ a b Roco, Mihail C. and Bainbridge, William Sims, eds. (2004). Converging Technologies for Improving Human Performance. Springer. ISBN 1-4020-1254-3.
{{cite book}}:|author=has generic name (help)CS1 maint: multiple names: authors list (link) - ↑ Agar, Nicholas (2004). Liberal Eugenics: In Defence of Human Enhancement. ISBN 1-4051-2390-7.
- ↑ Parens, Erik (2000). Enhancing Human Traits: Ethical and Social Implications. Georgetown University Press. ISBN 0-87840-780-4.
- ↑ Kringelbach ML, Jenkinson N, Owen SLF, Aziz TZ (2007). "Translational principles of deep brain stimulation". Nature Reviews Neuroscience. 8:623–635. PMID 17637800.
- ↑ Ford, Alyssa (May–June 2005). "Humanity: The Remix". Utne Magazine. Retrieved 2007-03-03.
- ↑ Mooney, Pat Roy (2002). "Beyond Cloning: Making Well People "Better"". Retrieved 2007-02-02.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Fukuyama, Francis (2002). Our Posthuman Future: Consequences of the Biotechnology Revolution. Farrar Straus & Giroux. ISBN 0-374-23643-7.
- ↑ Institute on Biotechnology and the Human Future. "Human "Enhancement"". Retrieved 2007-02-02.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Michael Hauskeller, Better Humans?: Understanding the Enhancement Project, Acumen, 2013, ISBN 978-1-84465-557-1.
- ↑ Good, Better, Best: The Human Quest for Enhancement Summary Report of an Invitational Workshop. Convened by the Scientific Freedom, Responsibility and Law Program. American Association for the Advancement of Science. June 1–2, 2006. Author: Enita A. Williams. Edited by: Mark S. Frankel.
- ↑ Hughes, James (2004). Citizen Cyborg: Why Democratic Societies Must Respond to the Redesigned Human of the Future. Westview Press. ISBN 0-8133-4198-1.
- ↑ U.S. Department of Health and Human Services.FDA approves implanted brain stimulator to control tremors. Retrieved October 18, 2006.
- ↑ 'Brain pacemaker' treats dystonia. KNBC TV, April 22, 2003. Retrieved October 18, 2006.
- ↑ FDA Approves Humanitarian Device Exemption for Deep Brain Stimulator for Severe Obsessive-Compulsive Disorder.
- ↑ National Institute of Neurological Disorders and Stroke. Deep brain stimulation for Parkinson's Disease information page. Retrieved November 23, 2006.
- ↑ Volkmann J, Herzog J, Kopper F, Deuschl G. "Introduction to the programming of deep brain stimulators". Mov Disord. 2002 17, S181–187. PMID 11948775.
- ↑ Starr PA, Martin AJ, Ostrem JL, Talke P, Levesque N, Larson PS. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy. J Neurosurg. 2010 Mar;112(3):479-90. doi: 10.3171/2009.6.JNS081161. PMID 19681683
- ↑ Deep Brain Stimulation, Department of Neurological Surgery, University of Pittsburgh. Retrieved May 13, 2008.