Wikijunior:The Elements/Structure
Structure[edit | edit source]

Protons, neutrons and electrons make up atoms. Because of this, they are called subatomic particles. Each type of atom can differ in the number of protons, neutrons, and electrons. According to the current electron cloud theory of atoms: there are two parts to the atom, the nucleus and the electron cloud. The nucleus is made up of protons and neutrons, and in turn each of these nucleons is made up of triples of even smaller particles called "quarks".
The nucleus is the center of the atom and contains the protons and neutrons. The nucleus is very small compared to the size of the electron cloud. This means there a massive amount of empty space around the nucleus. The protons and neutrons of an atom are in specific locations. Both protons and neutrons have a mass of about 1 amu (atomic mass unit) each. The atomic mass of an atom is thus the sum of the number of protons and neutrons. (Electrons are thousands of times lighter, so they aren't part of the calculation.)
The protons have a positive charge. The number of protons decides what element an atom is. For example, if an atom has one proton, that means it is a hydrogen atom; only hydrogen can have one proton. On the periodic table, the atomic number of the element is the same as its number of protons.
The neutrons have no charge, but they help stabilize the nucleus; if the positive charges of the protons were by themselves in the nucleus, they would repel each other and make the nucleus less stable. Atoms of the same element but with different numbers of neutrons called isotopes of each other. For example, hydrogen has three natural isotopes, one with no neutrons, one with one neutron, and one with two neutrons. They all have one proton and so they are all hydrogen, but because they differ in the number of neutrons they are different isotopes. Some isotopes are radioactive, which means that they decay, or release over time. A certain radioactive isotope of carbon, called carbon-14 (carbon with an atomic mass of 14) is used by paleontologists to discover the age of fossils. They can do this because they know the rate at which carbon-14 decays.
The outer part of the atom, the electron cloud, surrounds the nucleus. According to a theory called quantum mechanics, we never know the exact location or speed of a specific electron; we can only say the probability of it being anywhere. If we know exactly where the electron is, we don't know how fast it is going. If we know how fast it is going, likewise, we cannot determine where exactly it is located. An electron has a negative charge that is as powerful as a proton's positive charge. Some scientists used to think that electrons orbited the nucleus like planets around the sun, but we now know this isn't true. The electrons move somewhat randomly around the nucleus and are attracted to the positive charge of the protons in the nucleus.
Atoms = Building Blocks[edit | edit source]
Atoms are the basis of chemistry and everything in the Universe. You should start by remembering that matter is composed of atoms. Atoms and the study of atoms are a world unto themselves. We're going to cover basics like atomic structure and bonding between atoms. As you learn more, you can move to the biochemistry tutorials and see how atoms form compounds that help the biological world survive.
Electron Arrangement[edit | edit source]
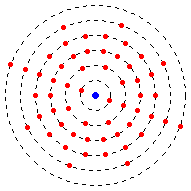
Electrons are organized in energy levels.
| Energy level | Electron capacity[1] |
|---|---|
| First | 2 |
| Second | 8 |
| Third | 18 |
| Fourth | 32 |
| Fifth | 50 |
| Sixth | 72 |
The energy levels don't have to be full for electrons to be in a higher energy level. For example, titanium has an electron configuration of 2-8-10-2. That means it has 2 electrons in the first energy level, 8 in the second, 10 in the third, and 2 in the fourth.
As explained before, some atoms only have a certain amount of electrons, so some elements don't have as many energy levels with electrons as others.
The outermost energy level is called the valence energy level. Likewise, electrons in the outermost energy level are called valence electrons. These electrons help atoms combine into molecules.