Structural Biochemistry/Secretins
There are many hormones within the human body which regulate and control bodily functions. Among them, one key member is Secretin. Secretins are hormones which regulate normal secretion into the duodenum and manipulate homeostasis throughout the human body. Duodenums are found in the intestinal gland found contained within the epithelial lining of the small intestine in the human body. Here, S cells, which are responsible for the creation of secretin, can be found. Secretins are found to participate in vital features of osmoregulation in several of the body's functional organs such as the kidney. A more specific feature for the functionality of secretins is their ability to regulate pH of the contents within the duodenum. This functionality can be carried out because the secretin hormones are able to manipulate specific secretion of gastric acid and control it's pH properties by using various chemical buffers. Typically, the chemical buffers used must be something the human body can have readily and not require additional intake of nutrients. The compound used by this the specific secretin to buffer pH then is bicarbonate. Bicarbonate can be found from the spindle-shaped cells called centroacinars within the pancreas. In addition to being discovered in the pancreas, bicarbonate can also be found in the intercalated ducts. An interesting fact about secretins are that they were the first hormone to be discovered and identified correctly.
A Biological and Histological Milestone[edit | edit source]
Secretins were first discovered in the early 1900s by two english physiologists, Ernest Starling and William Bayliss. The experimental design which for their project was to target how a nervous system responded and regulate digestion processes. Because of earlier discoveries, they knew that the pancreas was the main component in secreting compounds which aid in digestion. This process can occur as food enters the duodenum through the pyloric sphincter. The two scientists were able to contradict this earlier discovery during this experiment when they found that the nervous system, in fact, did not control digestive functions. Rather, they discovered substances now known as secretin produced in the intestines is the actual factor which controls the digestive system.
Biochemical Structure[edit | edit source]
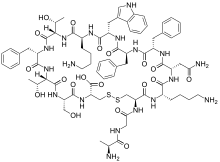
The chemical composition of Secretin involves several key components. The structure is first created initially with a precursor which contains the a spacer, the secretin hormone itself, an N-terminal signal peptide, and a C-terminal peptide which contains 72 amino acids. The entire precursor to the hormone is 120 amino acids long in total; however, the secretin portion is actually only between the residues of 28 and 54. In addition to the precursor peptide, there also contains a mature secretin peptide. This peptide is composed of 27 Amino Acid residues and is arranged in an linear sequence. It is characteristic in having a molecular mass of 3055. The 3-D structure of this mature linear secretin peptide shares its structure to some other common compounds. Similar to that of glucagon, glucose-dependent insulinotropic peptides, and vasoactive intestinal polypeptide, the secretin takes on the shape of a helix. This is most prominent in positions of amino acids five to thirteen. The amino acid breakdown of this helical chemical structure is as follows. Seven of the amino acid residues in mature secretin can also be found in the vasoactive intestinal polypeptides, fourteen can be found in similar placement as those found in glucagon, and ten amino acids residues in secretin can be found in corresponding positions in the glucose-dependent insulinotropic peptides. In addition to the precursor and mature secretin. Another special property of the secretin hormone is that it has an amidated carboxyl-terminal amino acid, valine.
Therefore, the corresponding sequence for the secretin hormone is: H2N–His-Ser-Asp-Gly-Thr-Phe-Thr-Ser-Glu-Leu-Ser-Arg-Leu-Arg-Asp-Ser-Ala-Arg-Leu-Gln-Arg-Leu-Leu-Gln-Gly-Leu-Val–CONH2.
Types of Secretin[edit | edit source]
T2SS: The Type II Secretion System helps regulate the amount of hydrolytic enzymes as well as expelling toxins from the periplasm outwards of the cell. Bacteria that have been known to carry T2SS was Escherichia coli and Vibrio cholera, bacteria which are known for cholera and diarrheal diseases. The toxins that are associated with those diseases have been correlated to the T2SS protein.
T4PS: The Type IV Pili System is a secretin that helps with the production and dismantling of extracellular fibers found on pathogenic/environmental bacteria. This secretin is made of many types of type IV pilin; T4aP and T4bP. These type IV pili are mainly responsible for motility, protein secretion, and attaching the secretin to cells. Some bacteria that have T4PS are Neisseria gonorrhoeae and pseudomonas aeruginosa.
T3SS: The Type III Secretion System is a pathway that transfers viral proteins to the cytoplasm. Also known as injectisomes, T3SS allows for pathogens to transfer effectors into the host and directly altering normal cell behavior. Pathogenic responses from T3SS could lead to physiological cell responses such as inflammation, phagocytosis, and even cell apoptosis. Bacteria that utilize T3SS is Salmonella, enteropathogenic Escherichia coli, and Shigella flexneri.
Physiology[edit | edit source]
Secretin hormones are first created in the S-cells. Specifically, in the cytoplasmic secretory granules portion of this cell. Furthermore, S cells can also be found in the mucous membranes which are linings covered in epithelium, a product which plays a key component in absorption and secretion. In addition, these linings can also be found in the duodenum as well as the jejunum portion of the smaller intestines. However, the S-cells and the secretin count in the small intestines are in smaller portions here.
Stimulus[edit | edit source]
Depending on differing species, secretin usually produce different responses based on the pH range of the environment of the duodenum. Generally, when the duodenal pH reaches an acidic function of around 2 to 5, the secretin hormone will be activated and released into circulation. Another case of secretin release could also occur within the intestinal lumen. However, both cases are in response to the pH change into the lower spectrum. In addition to pH control, the secretin secretion is can also be maximized when amino acid residue decomposition produces products which are contained in the mucosa of the smaller intestine, usually in the upper region.

Duodenums usually create their acidic environment based on the amount of hydrochloric acid(HCl) contained in their chambers. Masses of semi-digested food, or chyme, is jettisoned by the stomach and into the duodenum. As a result of this, hydrochloric acid is carried through the region of the stomach which connects the stomach and duodenum, called the pylorus sphincter. Once the HCl has reached the duodenum, it will acidify the environment and cause a pH drop, and in turn activate the release of secretin. Once secretin is finally released, it is used to target the glandular organ in the digestive and endocrine system, the pancreas. Once the pancreas receives a certain amount of insulin, the organ can then produce and expel a bodily fluid which is rich in bicarbonate. This solution then flows through the corresponding intestines. The pancreas needs to produce bicarbonate because the individual ions produced by this polyatomic ion can be used to neutralize the acid which is already present. With the bicarbonate present, the body can now utilize this to create a pH favorable environment so that it can carry out actions of the digestive enzymes in the intestinal tracts. A key feature of having bicarbonate is to neutralize the acid and prevent acid burns. The pancreas can also introduce bicarbonate as a response to the secretin by produce fatty acids and various bile salts. Both of these compounds can in turn produce bicarbonates as a product and be put in combination with the initial set of bicarbonate and placed in the small intestines. A method in which the secretion of secretin can be inhibited is by the addition of a class of drugs used to block the action of histamine in the cells on the stomach region. These are called the H2 receptor antagonists. These compounds function by reducing the gastric acid secretions. Due to the lowered amount of gastric acid in the duodenum, the pH cannot be maintained at a lowered level. Thus, once the pH of the duodenum has increased above 4.5-5, the secretin can no longer be produced and released. And the functions of the corresponding organs can be successfully shut down.
Function[edit | edit source]
The main function of the secretin hormone is to increase the bicarbonate solution from the bile as well as the pancreatic duct epithelium. The cells which are stationed in the pancreas have receptors which recognize secretin hormones once the secretin reaches its plasma membrane. Once the hormone has attached itself to the receptor, it can actively energize and produce adenylate cyclase activity. The adenylate cyclase activity is used to convert adenosine triphosphate, ATP, to Cyclic adenosine monophosphate or cAMP. In other words, an energy storing molecule to a messenger important in biological processes. It is worth noting that Cyclic AMP is used as a secondary messager to transmit signals throughout the cell and promote the increase in releasing more water carbonate ions. In addition, the production of more Cyclic AMP can be used to promote the growth and structurally stabilize the pancreas.

Further functions of secretin include counteracting the effects of an increase in blood glucose concentration spikes that is produced by the pancreas. When the pancreas decreases its release of insulin, the blood glucose concentration within the human body begins to spike haphazardly. By releasing secretin into the body, it can postpone and eliminate the effects of such blood concentration spikes by triggering the insulin release.
Another function which secretin takes place in the human body is that it can actively delay and stop the gastrin release from the stomach. Secretin can preform this function because it can reduce the acid secreted from the stomach and inbits the gastrin release from G cells. This functionality aids in neutralizing the pH of the products of digestion entering the duodenum. It has been shown that enzymes produce digestive functions at an optimal state that is a pH with slight basicity.
In addition, a function that secretin serves in the human body is that it can actively increase the pepsin secretion from cells in the stomach which releases pepsinogen, chymosin, and gastric lipase, or chief cells. This active stimulation of chief cells can help decompose proteins during the process of food digestion. Secretin can achieve this functionality because it stimulates the release of somatostatin release-inhibiting factor and glucagon.
Finally, the last function of secretin in addition to maintaining the additional acidic chyme passed through the duodenal organs, secretin also has other functions as well. One of these is that secretin ameliorates the effect the peptide hormone cholecystokinin and induce extra secretion of certain enzymes needed in digestion from the pancreas. Another effect produced by secretin in this function is that it can also increase bile produced within the gallbladder, a small organ which aids in fat digestion.
Uses[edit | edit source]
There are a variety of sectors in industry which secretin can be used. One unique function that secretin serves is that it can increase the pancreatic secretion of a human. This is extremely important in the medical field as scientists whom monitor pancreatic functions and testing must require the amplified effect produced the secretin hormone. Several methods can be used to introduce secretin into the body. Two main pathway in which secretin can be introduced to the human body is either through a direct injection into the duodenum or through a normal injection The result of certain secretin tests have been used to prove and provide insight into abnormalities in the pancreas. Finally, it has been shown that secretin can provide potential treatment to autism and other brain-based diseases.
Role in bacteria[edit | edit source]
Secretins assist in secreting proteins into the extracellular environment of gram-negative bacteria (which are bacteria that have the ability to gain resistance to antibiotics [9]). In bacteria, they make up three different, outer membrane channels. [10]
Type 2 Secretin system(T2SS)[edit | edit source]
The T2SS consists of three subassemblies: the outer membrane complex, a filamentous pseudopilus, and the inner membrane platform.These are responsible for secreting toxins from the periplasm (the area between plasma membrane and membrane bordering the cytoplasm [11]).
Secretion occurs in two steps. The proteins are produced with N-terminal signal peptides. This is followed by removal of the signal peptide along with the folding and release of the mature proteins into the periplasm. They may undergo further modifications before they are secreted across the membrane through the T2SS.[13]
Type 4 Pilin System (T4PS)[edit | edit source]
The T4PS help move the bacteria across surfaces without the using a flagella by assembling and disassembling the fibers on the surfaces of bacteria. These system consists of two subclasses of pilus: T4aP and T4bP. [10]
The fiber is a three-layered helical structure of alpha-helices surrounded by a beta-sheets. These two inner layers are covered with the C-terminal regions of the surface. The N-terminal amino acid sequence forms the innermost coil of alpha-helices. This hydrophobic packing and the flexibility of α-helices allow pili to bend and to adopt twisted or bundled conformations. The middle layer of β-sheet is continuous from one monomer to the next, and the β-sheet hydrogen bonding which provide much of the stability. It is generally believed that the pilus is assembled from its base, as a pool of pilin is found in the cell membrane. As there is no channel in the center of the fiber, assembly from the tip is excluded.[14]
Type 3 Secretin System (T3SS)[edit | edit source]
These are also known as injectisomes. They are used to transport bacterial effectors.[10]
It has a basal body, a needle structure, and a tip. [12]
The tip is secreted into the surrounding environment by the bacteria and comes in contact with host cells. The needle structure forms a pore in the host cell membrane. The effector protein can then pass into the host cell via needle.[12] This would eventual lead to the spread of the bacteria within the host.
Secretin Assembly[edit | edit source]
Secretins are generally produced in the outer membrane by a lipoprotein called pilotins. One known example is MxiM, a pilotin commonly found in T3SS. MxiM is composed mainly of B-sheets with one alpha-helix, combining together to form a cone-like structure. The hydrophobic portion of MxiM’s B-sheets act as the binding portion for lipids or itself (the N-terminus can bind with the C-terminus, making a circular-linked structure). After formation, the pilotin binds with the outer membrane and secretin is assembled. The way that the pilotin forms with each other (or with other lipids or pilotin) help dictate its structure, which thus resembles the function of the pilotin. Different structured pilotin thus lead to the formation of different secretin specific for different functions.
Another notable example would be the pilotin PilF, which is essential for the production of PilQ, a T4PS secretin commonly found in the bacterium P. aeruginosa. PilF often binds with another pilotin from a similar bacterium N. meningitides; PilW, allowing for the creation of a multimer secretin. With regards to function following form, both pilotins have a concave structure on the surface, which many researchers have argued could be a potential binding site for the production of T4aP necessary to create T4PS secretins.
Upon production of the different secretin systems by the pilotins, the secretion systems bind to the inner membrane of the cell and forms a secretin multimer. These secretin multimers are composed of many secretins that come together in order to form a specific secretin system. Examples of such secretin include GspCEpsC and GspMEspM, which are two fluorescent green proteins that are commonly found in the T2SS system. These two proteins have been known to help with localization of the secretin system inside the membrane as well as the actual production of the entire system as a whole.
References[edit | edit source]
5. http://special.edschool.virginia.edu/information/secretin.html
6. http://www.drugs.com/ppa/secretin.html
7. http://www.medicinenet.com/secretin/supplements-vitamins.htm
8. http://www.nlm.nih.gov/medlineplus/ency/article/003892.htm
9. http://www.cdc.gov/hai/organisms/gram-negative-bacteria.html
10. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3155655/
11. http://www.merriam-webster.com/dictionary/periplasm
12. http://www.nature.com/nchembio/journal/v8/n1/fig_tab/nchembio.741_F1.html
13. http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2958.2001.02403.x/full
14.http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2958.1999.01339.x/full
15. http://www.sciencedirect.com/science/article/pii/S0968000411000557