Structural Biochemistry/Lipids/Membrane Lipids
Membrane proteins rely on their interaction with membrane lipids to uphold its structure and maintain its functions as a protein. For membrane proteins to purify and crystallize, it is essential for the membrane protein to be in the appropriate lipid environment. Lipids assist in crystallization and stabilize the protein and provide lattice contacts. Lipids can also help obtain membrane protein structures in a native conformation. Membrane protein structures contain bound lipid molecules. Biological membranes are important in life, providing permeable barriers for cells and their organelles. The interaction between membrane proteins and lipids facilitates basic processes such as respiration, photosynthesis, transport, signal transduction and motility. These basic processes require a diverse group of proteins, which are encoded by 20-30% of an organism’s annotated genes. There exist a great number of membrane lipids. Specifically, eukaryotic cells have a very complex collection of lipids that rely on many of the cell’s resources for its synthesis. Interactions between proteins and lipids can be very specific. Specific types of lipids cane make a structure stable, provide control in insertion and folding processes, and help to assemble multisubunit complexes or supercomplexes, and most importantly, can significantly affect a membrane protein’s functions. Protein and lipid interactions are not sufficiently tight, meaning that lipids are retained during membrane protein purification. Since cellular membranes are fluid arrangements of lipids, some lipids affect interesting changes to membrane due to their characteristics. Glycosphigolipids and cholesterol tend to form small islands within the membranes, called lipid rafts, due to their physical properties. Some proteins also tend to cluster in lipid raft, while others avoid being in lipid rafts. However, the existence of lipid rafts in cells seems to be transitory. Recent progress in determining membrane protein structure has brought attention to the importance of maintaining a favorable lipid environment so proteins to crystallize and purify successfully. Lipids assist in crystallization by stabilizing the protein fold and the relationships between subunits or monomers. The lipid content in protein-lipid detergent complexes can be altered by adjusting solubilisation and purification protocols, also by adding native or non-native lipids.
There are three type of membrane lipids: 1. Phospholipids: major class of membrane lipids. 2. glycolipids. 3. Cholesterols. Membrane lipids were started with eukaryotes and bacteria.
Types of Membrane Lipids[edit | edit source]
Lipids are often used as membrane constituents. The three major classes that membrane lipids are divided into are phospholipids, glycolipids, and cholesterol. Lipids are found in eukaryotes and bacteria. Although the lipids in archaea have many features that are related to the membrane formation that is similar with lipids of other organisms, they are still distinct from one another. The membranes of archaea differ in composition in three major ways. Firstly, the nonpolar chains are joined to a glycerol backbone by ether instead of esters, allowing for more resistance to hydrolysis. Second, the alkyl chains are not linear, but branched and make them more resistant to oxidation. The ability of archaeal lipids to resist hydrolysis and oxidation help these types of organisms to withstand the extreme conditions of high temperature, low pH, or high salt concentration. Lastly, the stereochemistry of the central glycerol is inverted. Membrane lipids have an extensive repertoire, but they possess a critical common structural theme in which they are amphipathic molecules, meaning they contain both a hydrophilic and hydrophobic moiety.
Although each membrane lipid is distinct there are several attributes that are universal. Membrane lipids are all closed bodies or boundaries separating substituent parts of the cell. The thickness of membranes is usually between 60 and 100 angstroms. These bodies are constructed from non-covalent assemblies. Their polar heads align with each other and their non-polar hydrocarbon tails align as well. The resulting stability is credited to hydrophobic interaction which proves to be quite stable due to the length of their hydrocarbon tails. The resulting structure is termed a lipid bilayer which mediates molecules that enter or leave the cell. Sugars are also a key structural component in cells by attaching themselves to lipids forming glycolipids which are used for identification of the cell by other molecules. Signaling molecules read the sugar patterns in glycolipids which then the glycolipids either allow or refuses entry of the cell. This process helps tissue and organ growth within the body. Phospholipids are another important component to such processes.
There are several other universal characteristics of membrane lipids such as they are electrically polarized attributing a negative charge within the membrane. This plays a role in many functions of the cell such as the transport of energy and the conversion of energy. Also, lipid membranes are asymmetric and fluid structures. Biological membranes lack symmetry elements and will diffuse without stable conditions. Lastly, lipid membranes have unique functions. These specific functions are due to membrane proteins
Phospholipids[edit | edit source]

The major class of membrane lipids are the phospholipids. They are abundant in all biological membranes. Phospholipds are made from four components: one or more fatty acids, a platform to which the fatty acids are attached, a phosphate, and an alcohol attached to the phosphate. The fatty acid portion provides the hydrophobic barrier found in lipids, where as the rest of the molecule has a hydrophilic property, enabling interaction with the aqueous environment. Phosopholipds are built upon a foundation of glycerol, a three-carbon alcohol, or sphingosine. Phospholipids which are derived from glycerol are also known as phosphoglycerides, which consist of a glycerol backbone where two fatty acid chains and a phosphorylated alcohol are attached. The major phosphoglycerides come from phosphatidate through the formation of an ester bond between the phosphate group of phosphatidate and the hydroxyl group of one of several alcohols. Sphingomyelin is a phospholipid found in membranes that is not derived from glycerol. The backbone in sphingomyelin, however, is sphingosine, which is an amino alcohol which contains a long, unsaturated hydrocarbon chain.
Phospholipids have a very important property of separating compartments. For example, in evolution, it was very important in dealing with the RNA World Hypothesis. If RNA did not have a phospholipid bilayer, they would not have been able to contain all their chemical and mechanistic reactions in a certain space, and it would have been very hard for these RNA to survive without being disrupted by other arbitrary, outside reactions. Thus, phospholipid bilayers played a significant role in the production and survival of these RNA molecules.
Phospholipids have many unique functions. For example, they can function as a reservoir of intracellular protein messengers such as phosphoinositol biphosphate. Phosphoinositol biphosphate is one of the most important secondary messengers in the cell signaling pathway of human. Also, they anchor proteins to cells. This many determines the specific function of that lipid membrane. Phospholipids have a number of other functions making them the most abundant membrane lipid. These include energy storage, cellular shape, and it is a source of acetylcholine. Acetylcholine is a commonly occurring neurotransmitter found in both the peripheral nervous system (PNS) and the central nervous system (CNS).

Glycolipids[edit | edit source]
As the name implies, glycolipids are simply sugar-containing lipids. Glycolipds are typically composed of short, branched chains with less than 15 sugar units. The glycolipids in animal cells come from sphingosine, similar to those in sphingomyelin. As in sphingomyelin, the amino group of the sphingosine backbone is acylated by a fatty acid. Glycolipids have a unit that is linked to the primary hydroxyl group of the sphingosine backbone, which differentiates it from sphingomyelin. In glycolipids, one or more sugars are attached to this group. Glycolipids are arranged in an asymmetric manner with the sugar residues always on the extra cellular side of the membrane. The simplest glycolipid is called cerebroside which contains a single sugar residue. The sugar could be either glucose or galactose. Complex glycolipids, for example gangliosides, contain a branched chain of as many as seven sugar residues.
Glycolipids serve several important functions within the cell. These include shape, specific function, fuel storage, and a number of cellular tasks. For example, glycolipids flank from the extra cellular side of the membrane and serve as a marker for cellular recognition. The information many times tells a cell whether to grow, divide, or do nothing. Likewise, these markers can also be the antigens that correspond to blood type. Other molecules that serve as antigens to blood type are glycoproteins.
Cholesterol[edit | edit source]
Cholesterol is a lipid with a structure quite different from that of phospholipids. It is a steroid built from four linked hydrocarbon rings. A hydrocarbon tail is at one end of the steroid while a hydroxyl group is attached to the other end. The orientation of the molecule in a membrane is parallel to the fatty acid chains of the phospholipids, allowing the hydroxyl group to interact with the head group of phospholipids within the proximity. Cholesterol is absent from prokaryotes, but found in varying degrees in all animal membranes. Almost 25% of membrane lipids in certain nerve cells is made of cholesterol. Furthermore, cholesterol is also present in hormones found in the body. However, cholesterol is essentially non-existent from some intracellular membranes. Cholesterol contains four cycloalkane rings and OH group attaches at one end. Cholesterol is functioned as cell signaling and stays outside of the membrane. Cholesterol although it has a negative reputation has a number of important functions. There are two types of cholesterol HDL and LDL known as “good” and “bad” cholesterol, respectively. Low density lipoproteins (LDL) are known as bad because it tends to thicken the walls of the heart and arteries with plaque. This can lead to heart attacks, strokes, and a number of other health concerns. High density lipoprotein (HDL) is known as good cholesterol because it seems to do the opposite. It is believed that HDL cholesterol actually takes cholesterol from the heart back to the liver where it can be broken down and passed through the body. Cholesterol has a number of other functions important to the field of biochemistry. It is used to build membrane, supply the body with fat, and it also synthesizes hormones necessary for bodily function. Although it is necessary to find this membrane lipid in much lower quantities than phospholipids it serves several significant functions.
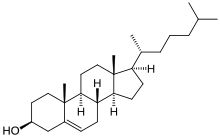
The sterol cholesterol is a major constituent of animal plasma membranes but is absent from prokaryotes. The fused ring system of cholesterol means that it is more rigid than other membrane lipids.As well as being an important component of membranes, cholesterol is the metabolic precursor of the steroid hormones. Plants contain little cholesterol but have instead a number of other sterols, mainly stigmasterol and beta-sitosterol which differ from cholesterol only in their aliphatic side-chains.
Membrane Lipids[edit | edit source]
Lipid Vesicles[edit | edit source]
Lipid vesicles, also known as liposomes, are vesicles that are essentially aqueous vesicles that are surrounded by a circular phospholipid bilayer. Like the other phospholipid structures, they have the hydrocarbon/hydrophobic tails facing inward, away from the aqueous solution, and the hydrophilic heads facing towards the aqueous solution. These vesicles are structures that form enclosed compartments of ions and solutes, and can be utilized to study the permeability of certain membranes, or to transfer these ions or solutes to certain cells found elsewhere.
Liposomes, with a diameter of roughly 500 A, are formed by dispelling a suitable lipid in an aqueous environment, and then sonicating the solution with high-frequency sound waves, for example, which aid these molecules to form a dispersed set of closed vesicles that are all nearly identical in size. Larger vesicles can be formed by placing a phospholipid in a solution containing both organic and aqueous solvents and then slowly evaporate the organic solvent. When this occurs with ions and solutes in the solution as well, that is when these compartments can entrap these ions and solutes in their lipid vesicles. The vesicles that contain the ions or solutes can then be separated by gel-filtration chromatography or dialysis. The permeability of a molecule can be obtained using this idea and then measuring the rate at which the molecule moves from the inner compartment of the vesicle to the outer solution.
The formation of liposomes is extremely useful in drug transport and delivery. Since drugs cannot readily diffuse through cell membranes, by creating liposomes in a solution containing a specific drug, the drug can enter into the cell by fusion of the liposome bilayer with the cell bilayer, delivering the liposome's contents. This technique can also be used to deliver DNA.
Liposomes as vesicles can serve various clinical uses. Injecting liposomes containing medicine or DNA (for gene therapy) into patients is a possible method of drug delivery. The liposomes fuse with other cells' membranes and therefore combine their contents with that of the patient's cell. This method of drug delivery is less toxic than direct exposure because the liposomes carry the drug directly to cells without any unnecessary intermediate steps. Long-circulating liposomes are more concentrated in regions of high blood circulation(like tumors and inflamed areas), and the selective fusion exhibited by liposomes allows them to be able to target specific types of cells. This is a very useful tool in designing carefully-controlled drug delivery techniques.
Lipid Bilayer[edit | edit source]
Because of the hydrophobic interactions among several phospholipids and glycolipids, a certain structure called the lipid bilayer or bimolecular sheet is favored. As mentioned earlier, phospholipids and glycolipids have both hydrophilic and hydrophobic moieties; thus, when several phospholipids or glycolipids come together in an aqueous solution, the hydrophobic tails interact with each other to form a hydrophobic center, while the hydrophilic heads interact with each other forming a hydrophilic coating on each side of the bilayer.
The cellular bilayer is not a rigid and clearly defined structure separating the intracellular and extracellular environments. Realistically, there is significant fluid motion of individual lipid headgroups and their aliphatic chains within the plane of the bilayer. This is due to the low torsional angle barriers within the structures of the lipids and to the steric hindrances that result from the cis-double bounds of some aliphatic chains.[1]
Due to the large variety lipids that can exist in a single cell, regions of cellular membranes will comprise of heterogeneous mixtures of lipids and membrane proteins.[2] The unique interactions within these heterogeneous regions are the bases of the function of the cellular membrane.

This lipid bilayer formation is spontaneous because of the hydrophobic interactions and energetically favorably structure. Other intermolecular forces such as Van der Waals, which hold the hydrophobic tails together, and hydrogen bonding, which bind the hydrophilic heads with water, help stabilize the lipid bilayer structure. Because of these interactions, the lipid bilayer inherits unique properties. The lipid bilayer have "extensive" properties, and can enclose and form compartments. Lastly, they can also recover quickly if there is a hole in the lipid bilayer, due to energetic reasons. However, phospholipids and glycolipids do not form micelles like fatty acids do because phospholipids and glycolipids have two fatty acids chains and are too big to form the interior of the micelle.
In aqueous solution, amphipathic molecules will orientate themselves in such a way as to prevent the hydrophobic region coming into contact with water molecules. In the case of those fatty acid salts which contain only one fatty acid chain (such as sodium palmitate, a constituent of soap), the molecules form a spherical micellar structure (diameter usually <20 nm) in which the hydrophobic fatty acid chains are hidden inside the micelle and the hydrophilic headgroups interact with the surrounding water molecules. Because the two fatty acid chains of phospholipids are too bulky to fit into the interior of a micelle, the favored structure for most phospholipids in aqueous solution is a two-dimensional bimolecular sheet or lipid bilayer.
Asymmetry of Lipid Bilayer[edit | edit source]
Plasma membranes are structurally and functionally asymmetric. There are different compositions and ratios of phospholipids inside and outside of lipid bilayer. In the plasma membrane, phosphatidylcholine and sphingomyelin are found in the outer monolayer (outer leaflet) but phosphatidylserine, phosphatidylethanolamine and the phosphatidylinositols are found in the inner monolayer (inner leaflet). Cholesterols are found in both leaflets.

The distribution of lipids between the lipid bilayer often changes to induce biological effect. A good example is that platelet moves phosphatidylserine to the outer leaflet to enable the ability of blood clotting. Phosphatidylserine is also an important signal for programmed cell death. In some cells, the presence of phosphatidylserine on the outer surface of cell membrane signals the immune system to destroy the marked cell. The asymmetry property of cell membrane enables cell to have multiple biological functions.
Membrane proteins are also inserted into the membrane in an asymmetric way.
In a lipid membrane, there are proteins embedded in them. The mass ratio of the lipid molecules and the proteins that are embedded in them ranges from 1:4 to 4:1. There are two types of proteins in lipid bilayer: integral and peripheral membrane proteins. Integral membrane proteins traverse the lipid bilayer meaning they interact extensively with the hydrophobic region (hydrocarbon region) of the lipid bilayer. Peripheral membrane proteins are usually attached to surfaces of integral proteins; therefore, they are on both faces of lipid bilayer. Peripheral membrane proteins interact with the hydrophilic polar head groups of the lipid molecule. Some peripheral membrane proteins can only be found outside the membrane or inside the membrane. This contributes the asymmetric property of membrane.

Protein Functions in Membrane[edit | edit source]
Proteins are responsible for the active transport of ions and larger molecules in and out of the cell membrane. They are classified as either integral or peripheral. Both forms of the protein are embedded in the lipid bilayer thru hydrophobic interactions. A typical integral protein spans the width of the lipid bilayer. They usually consist of many alpha helices, with their hydrophobic parts pointing outwards, into the membrane's hydrophobic sea of hydrocarbon tails. Alpha helices are non-polar and uncharged. This can be used to identify likely membrane - spanning regions. Free - energy can be estimated when a helical segment is transferred from the interior of the membrane to water. Each amino acid residue has a specific free energy change thus allowing us to know what the alpha - helices is made of. Free - energy chance for each window can be plotted against the first amino acid to create a hydropathy plot. Peaks of greater than 84kJ/mol have the potential to be transmembrane helices. When integral proteins are made of beta pleated sheets, they typically fold into a cylinder, once again, with the hydrophobic ends pointing out and the hydrophilic ends pointing in. These are called channel proteins and they usually work well as a form of ion transport because the hydrophilic core will allow charged particles to go thru. A peripheral protein is bound to the membrane by electrostatic and hydrogen-bond interactions with the head groups of the lipids. These interactions can be interrupted by the change of salt concentration and pH.
Built from the concept of integral and peripheral proteins, one biological significance comes from the structure of an archaeic protein called, bacteriorhodopsin. Bacteriorhodopsin consists of seven alpha helices that have a majority of nonpolar amino acids and few charged amino acids. The protein is a integral protein, which means it interacts with the hydrocarbon chains in the membrane. Having nonpolar alpha helices allows this protein to be bound within the membrane. The purpose of being an integral protein is so that it allows the protein to generate a proton gradient by transporting protons from inside the cell to the outside of the cell. The purpose of the gradient is to allow the formation of ATP.
Each type of membrane has its specific types of lipids and more importantly proteins. The protein composition of different membranes varies even more than their lipid composition. The vertebrate retina rod cell has one part of membrane specialized for the reception of light. This specific part of membrane contains the protein, light-absorbing glycoprotein rhodopsin, which constitute 90% of the proteins of that specific part of the membrane. In a less-specialized plasma membrane like Escherichia coli membrane, there are hundreds of different proteins, including channels, transporters, and enzymes that manage metabolism, lipid synthesis, cell division and more functions.
Generally speaking, there is no specific percentage or ratio of proteins to lipid molecule. Each cell has different protein and lipid composition corresponding to its functions. Therefore, the ratio of protein to lipid molecule can range from 1:4 to 4:1.
Lipid Rafts[edit | edit source]
Structure
Lipid rafts are unique sections of membrane that have a high number of glycolipids, cholesterols, and certain proteins. The existence of lipid rafts was first proposed in 1988 by Simons and van Meer, but another set of structures known as "caveolae" were first observed much earlier. Caveolae are small depressions on the cell surface that are considered a type of membrane raft, these were named "caveolae intracellulare" (Yamada, 1955). After much debate by world renowned researchers, most in the scientific community accept that these rafts exist; however, it is unclear to most how to classify these rafts. Currently, the classification system includes three distinct groups; caveolae, glycosphingolipid enriched membranes (GEM), and polyphospho inositol rich rafts. There is also the possibility that there are inside rafts (PIP2 rich and caveolae) and outside rafts (GEM).
Most of these rafts are very highly ordered and rigid as a result of a high concentration of glycosphingolipids that characterize this portion of the membrane. The lipids included in the raft are tightly packed and extended, so they also often include other lipids with long, straight, acyl chains.
Function
Lipid rafts in the membrane have thought to carry out many important membrane functions from cholesterol transport, to endocytosis and signal transduction. Several experiments have shown that lipid rafts play an essential role in the signal transduction pathway. Although it has been proposed that the caveolae was an integral part of endocytosis, but recent data has disproved this theory. Instead caveolae are very stable regions of membranes that are not involved in endocytosis (Thompsen et al., 2002).
Rafts and the Cytoskeleton
Some actin binding proteins have been shown to bind to polyphosphoinositides and to be regulated by them by a variety of protein domains such as PH, PX and ENTH. It follows that some ABPs are suggested to link the actin cytoskeleton and PIP2-enriched rafts. One of these is gelsolin, a Ca2+, pH and polyphosphoinositide regulated actin capping and severing protein, that breaks into rafts isolated biochemically from the brain (Fanatsu et al., 2000). GEMs are also thought to link to the actin cytoskeleton through ABPs, specifically ERM proteins through EBP50, a protein that binds members of the ERM proteins through the ERM C-terminus (Brdickova et al., 2001).
References[edit | edit source]
von Heijne, G and Rees,D (2008). Current Opinion in Structural Biology. Elsevier Ltd.
Berg, Biochemistry, 6th Edition
Campbell and Reece, Biology, 7th Edition
Lehninger, Principles of Biochemistry, 4th edition
University of Edinburgh [2]
