Structural Biochemistry/Enzyme/Reversible Inhibitors
Enzyme inhibitors are molecules or compounds that bind to enzymes and result in a decrease in their activity. An inhibitor can bind to an enzyme and stop a substrate from entering the enzyme's active site and/or prevent the enzyme from catalyzing a chemical reaction. There are two categories of inhibitors.
- irreversible inhibitors
- reversible inhibitors
Inhibitors can also be present naturally and can be involved in metabolism regulation. For example. negative feedback caused by inhibitors can help maintain homeostasis in a cell. Other cellular enzyme inhibitors include proteins that specifically bind to and inhibit an enzyme target. This is useful in eliminating harmful enzymes such as proteases and nucleases.
Examples of enzymes inhibitors include poisons and many different types of drugs.
Reversible inhibitors can bind to enzymes through weak non-covalent interactions such as ionic bonds, hydrophobic interactions, and hydrogen bonds. Because reversible inhibitors do not form any chemical bonds or reactions with the enzyme, they are formed rapidly and can be easily removed; thus the enzyme and inhibitor complex is rapidly dissociated in contrast to irreversible inhibition.
Examples of reversible inhibition:
- competitive inhibition (Raises Km only)
- uncompetitive inhibition (Lowers Vmax and Km)
- noncompetitive inhibition (Lowers Vmax only)
Examples of irreversible inhibition:
- group specific: reacts only to certain chemical group.
- reactive substrate analogs (affinity label): inhibitor that are structurally similar to the substrate and will bind to active site.
- mechanism-based inhibitors (suicide inhibitors): enzymes converts the inhibitor into a reactive form within active site.
Competitive inhibition can be overcome by increasing the concentration of substrate while uncompetitive and noncompetitive inhibition cannot.
Irreversible Inhibitors[edit | edit source]
Irreversible inhibitors covalently bind to an enzyme, cause chemical changes to the active sites of enzymes, and cannot be reversed. A main role of irreversible inhibitors include modifying key amino acid residues needed for enzymatic activity. They often contain reactive functional groups such as aldehydes, alkenes, or phenyl sulphonates. These electrophilic groups are able to react with amino acid side chains to form covalent adducts. The amino acid components are residues containing nucleophilic side chains such as hydroxyl or sulfhydryl groups such as amino acids serine, cysteine, threonine, or tyrosine.
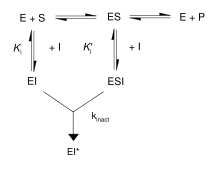
First, irreversible inhibitors form a reversible non-covalent complex with the enzyme (EI or ESI). Then, this complex reacts to produce the covalently modified irreversible complex EI*. The rate at which EI* is formed is called the inactivation rate or kinact. Binding of irreversible inhibitors can be prevented by competition with either substrate or a second, reversible inhibitor since formation of EI may compete with ES.
In addition, some reversible inhibitors can form irreversible products by binding so tightly to their target enzyme. These tightly-binding inhibitors show kinetics similar to covalent irreversible inhibitors. As shown in the figure, these inhibitors rapidly bind to the enzyme in a low-affinity EI complex and then undergoes a slower rearrangement to a very tightly bound EI* complex. This kinetic behavior is called slow-binding. Slow-binding often involves a conformational change as the enzyme "clams down" around the inhibitor molecule. Some examples of these slow-bindinginhibitors include important drugs such as methotrexate and allopurinol.
Reversible Inhibitors[edit | edit source]
Reversible inhibitors bind non-covalently to enzymes, and many different types of inhibition can occur depending on what the inhibitors bind to. The non-covalent interactions between the inhibitors and enzymes include hydrogen bonds, hydrophobic interactions, and ionic bonds. Many of these weak bonds combine to produce strong and specific binding. In contrast to substrates and irreversible inhibitors, reversible inhibitors generally do not undergo chemical reactions when bound to the enzyme and can be easily removed by dilution or dialysis.
There are three kinds of reversible inhibitors: competitive, noncompetitive/mixed, and uncompetitive inhibitors.
- Competitive inhibitors, as the name suggests, compete with substrates to bind to the enzyme at the same time. The inhibitor has an affinity for the active site of an enzyme where the substrate also binds to. This type of inhibition can be overcome by increasing the concentrations of substrate, out-competing the inhibitor. Competitive inhibitors are often similar in structure to the real substrate.
- Uncompetitive inhibitors bind to the enzyme at the same time as the enzyme's substrate. However, the binding of the inhibitor affects the binding of the substrate, and vice-versa. This type of inhibition cannot be overcome, but can be reduced by increasing the concentrations of substrate. The inhibitor usually follows an allosteric effect where it binds to a different site on the enzyme than the substrate. This binding to an allosteric site changes the conformation of the enzyme so that the affinity of the substrate for the active site is reduced.
- Non-competitive inhibitors bind to the other sites (Allosteric Sites), not the active site, and stops the enzyme's activity by changing the shape of the active site (caused by disruption to the normal arrangement of hydrogen bonds and weak hydrophobic interactions holding the enzyme molecule together in its 3D shape. This distortion ripples to the active site making it unsuitable) . Therefore, concentration of the substrate is meaningless unlike in competitive inhibition.
Few examples of Reversible inhibitors:
Acetylcholinesterase inhibitors: Often abbreviated AChEI or anti-cholinesterase it is a chemical that inhibits the enzyme Acetylcholinesterase from breaking down acetylcholine. This ultimately leads to increase in both the level and longevity of action of the neurotransmitter acetylcholine.
Reversible inhibitor of monoamine oxidase A(maoA): maoA inhibitors compromise of a wide range of natural as well as psychiatric drugs that inhibits the enzyme monoamine oxidase temporarily and reversibly. maoA inhibitors are most commonly used to fight depression and dysthymia.
Quantitative Description of Reversible Inhibitors[edit | edit source]

Most reversible inhibitors follow the classic Michaelis-Menten scheme, where an enzyme (E) binds to its substrate(S) to form an enzyme-substrate complex (ES). km is the Michaelis constant that corresponds to the concentration of the substrate when the velocity is half the maximum. Vmax is the maximum velocity of the enzyme.
- Competitive inhibitors can only bind to E and not to ES. They increase Km by interfering with the binding of the substrate, but they do not affect Vmax because the inhibitor does not change the catalysis in ES because it cannot bind to ES.

-
Diagram showing competitive inhibition
-
Competitive inhibition can also be allosteric, as long as the inhibitor and the substrate cannot bind the enzyme at the same time
-
Another possible mechanism for allosteric competitive inhibition
- Uncompetitive inhibitors can only bind to the ES complex. Therefore, these inhibitors decrease Km because of increased binding efficiency and decrease Vmax because they interfere with substrate binding and hamper catalysis in the ES complex.

- Mixed inhibitors can bind to either E or ES complex, but have a preference for one or the other. This can either increase or decrease Km, respectively. Both cause a decrease in Vmax.
- Non-competitive inhibitors have identical affinities for E and ES. They do not change Km, but decreases Vmax.


-
Illustration of a possible mechanism of non-competitive or mixed inhibition



