Structural Biochemistry/Cell Organelles/Golgi Apparatus
Golgi apparatus, also known as the Golgi complex, golgi body, or simply golgi, is an organelle found in most eukaryotic cells. The golgi can be thought of as the "packaging, modifying and shipping warehouse" of the cell.
Overview[edit | edit source]

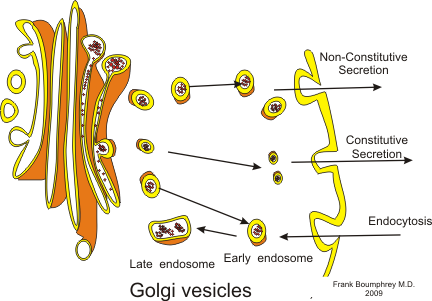
The Golgi apparatus, also called the Golgi complex, is commonly found in eukaryotic cells. The Golgi complex can be identified by its unique structure which some say looks like a maze, but in fact the structure is made of stacks of flattened membranous sacs, or cisternae. Unlike the cisternae of the endoplasmic reticulum or ER, these membranes are not connected. The Golgi apparatus is responsible for the processing and packaging of protein and lipids, as well as processing proteins for secretion. The Golgi apparatus is often thought of as a post office; through the process of protein glycosylation, sugars are added to the protein which dictate to where the protein should travel. N-linked glycosylation is begun in the endoplasmic reticulum but is continued in the golgi complex. O-linked glycosylation is solely done in the golgi complex. After proteins have been modified in the golgi apparatus, they are usually sent to either the lysosomes, secretory granules, or plasma membrane depending on the signals encoded within the protein sequence and structure. For this reason, Golgi complex is recognized as "the major sorting center" of the cell.
Golgi Apparatus as an Independent Organelle[edit | edit source]
Researchers in Yale show that the Golgi Apparatus is not just an outgrowth of another organelle, the endoplasmic reticulum, but an independent organelle on its own. The Golgi Apparatus is an organelle within a cell that has its own autonomy and so must grow and divide to keep pace with the growth and division that the cell inhibits. It has been found that even without the stacks of membrane compartments where secretory proteins pass, the Golgi Apparatus still functions. Proteins referred to as matrix proteins that form a scaffold have been found to be responsible for its growth, division and partitioning.
The Golgi Apparatus has been observed to grow, divide and be inherited like other organelles as has been observed in the regrowth of the mother Golgi Apparatus in daughter cells with the use of matrix proteins.
History[edit | edit source]
The Golgi was discovered by Italian physician Camillo Golgi in 1898 during an investigation of the nervous system. [1] Although the discoverer denoted the structure as the "internal reticular apparatus," scientists changed it to the "golgi complex" in 1910. Few doubted the discovery, claiming that the organelle was a mere illusion created by the optical instruments used that discovered them. Yet, soon after the advent of modern microscopes circa 1900's, scientists alike confirmed the presence of the Golgi.
Structure[edit | edit source]
The Golgi complex is made several flattened membranes sacs, but can be ultimately divided into two sections: the Cis Golgi and the Trans Golgi Network (TGN). The Cis Golgi functions as the receiving end for newly synthesized proteins from the lumen of the Endoplasmic Reticulum (ER). Vesicles containing proteins from the ER merge with the Cis golgi allowing the proteins to enter the Golgi complex. As the Cis golgi receives proteins from the ER, the proteins then begin their modification moving along membrane to membrane towards the TGN. At the other end of the golgi complex, the newly modified protein arrives at the TGN where it is then send off to different parts of the cell via a transport vesicle.
Origin of Golgi Apparatus[edit | edit source]
There are two prevailing theories as to the formation of the Golgi apparatus. The vesicular shuttle model postulates that Golgi cannot be made from scratch and that the vesicles of the endoplasmic reticulum are sent to the pre-existing Golgi. On the other hand, the cisternae maturation model suggests that vesicles from the ER fuse together to form the Golgi and as proteins are processed and mature they create the next Golgi compartment. New data suggest that perhaps neither model is completely correct. This will likely lead to yet another model. [2]
Cisternae Maturation Model[edit | edit source]
On the other side of the debate is Jennifer Lippincott-Schwartz of the National Institute of Child Health and Human Development (part of the National Institutes of Health) in Bethesda, Maryland. She says that the Golgi makes itself from scratch. According to her theory, packages of processing enzymes and newly made proteins that originate in the ER fuse together to form the Golgi. As the proteins are processed and mature, they create the next Golgi compartment. This is called the cisternae maturation model.
In this model, the cisternae of the Golgi apparatus move by being built at the cis face and destroyed at the trans face. The vesicles from the endoplasmic reticulum fuse together to form a cisterna at the cis face and this cisternae would appear to move through the Golgi stack when a new cisterna is formed at the cis face. This model is supported by the fact that structures larger than the transport vesicles were observed microscopically to progress through the Golgi apparatus. In summary, packages of processing enzymes and new proteins originating in the ER fuse together to form the Golgi and as the proteins are processed and mature, the next Golgi compartment is created. Vesicular transport is one of models of Golgi apparatus that says that Golgi cannot make from scratch and that vesicles in the ER are sent to pre-existing Golgi. [3]
Vesicular Shuttle Model[edit | edit source]
On one side of the debate is Graham Warren of Yale University School of Medicine in New Haven, Connecticut, who argues that the Golgi is an architectural structure that cannot be made from scratch. He believes that newly made proteins are packaged in the rough ER and are sent for further processing to a pre-existing structure (the Golgi) that is made up of different compartments. This is called the vesicular shuttle model.
This model views the Golgi apparatus as a very stable organelle that is divided into compartments in the cis to trans direction. Membrane bound carriers transport material between the endoplasmic reticulum and the different compartments of the Golgi. Experimental evidence shows the abundance of small vesicles in close proximity to the Golgi apparatus. Actin filaments direct the vesicles by connecting packaging proteins to the membrane to ensure that they fuse with the correct compartment. To summarize, newly made proteins are packaged in the rough ER and are sent for processing to a pre-existing structure known as the Golgi which is made up of different compartments.[4]
Vesicle Transport[edit | edit source]
As soon as modified proteins reach the Trans Golgi Network (TGN), the proteins are separated into several different transport vesicles for different areas of the cell.
| Vesicle Type | Description |
|---|---|
| Exocytotic vesicles | These vesicles contain protein that are to be sent outside the cell membrane. These vesicles fuse with the plasma membrane, releasing their contents outside the cell. This process is called constitutive secretion. |
| Secretory vesicles | These vesicles are also to be sent outside the cell membrane. However what differs these from exocytotic vesicles is that secretory vesicles need a signal before they are released. When the signal is given, they will fuse with the plasma membrane to release the contents. This process is called regulated secretion. |
| Lysosomal vesicles | These vesicles contain protein that are sent to the lysosome to be digested. |
Transport Mechanism[edit | edit source]
The transport mechanism that proteins use to progress through the Golgi apparatus is still not clear yet, but there are a number of hypotheses that currently exist. Up until recently, the vesicular transport mechanism had been the favored mechanism for transport but there is now more evidence that supports the Cisternal maturation. The two models may work in conjunction with one another rather than being mutually exclusive. This is sometimes referred to as the combined model.
Glycosylation[edit | edit source]
This is the process of adding sugar to a molecule. Glycosylation happens in the golgi apparatus and in the endoplasmic reticulum. Glycosylation can only happen when an amino acid group with a hydroxyl (-OH) attaches to a sugar molecule. Through a condensation reaction (water leaving) a sugar is added to the amino acid.
References[edit | edit source]
- Berg, Jeremy M., John L. Tymoczko, and Lubert Stryer. Biochemistry. 6th ed. New York: W.H. Freeman and Company, 2007. Print.
- http://opa.yale.edu/news/article.aspx?id=3365
- Inside the cell, U.S. Department of Health and Human Services
- Viadiu, Hector
- Reece, Jane (2011). Biology. Pearson. ISBN 978-0-321-55823-7.
{{cite book}}: Text "coauthors+ Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson" ignored (help) - Machalek M Alisa. http://publications.nigms.nih.gov/insidethecell/insidethecell.pdf "Inside the Cell" The National Institute of General Medical Sciences (2010): 36-37.
- http://en.wikipedia.org/wiki/Golgi_apparatus
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/9865849
- ↑ U.S. Department of Health and Human Services. Inside the Cell. September 2005.<http://www.nigms.nih.gov>.
- ↑ U.S. Department of Health and Human Services. Inside the Cell. September 2005.<http://www.nigms.nih.gov>.
- ↑ U.S. Department of Health and Human Services. Inside the Cell. September 2005.<http://www.nigms.nih.gov>.