Structural Biochemistry/Proteins/Introduction
Proteins are one of the most important macromolecules in living organisms that have many functions for biochemical processes. They are consisting almost entirely of carbon, hydrogen, oxygen, and nitrogen. The protein is a polymer of multiple monomer units called amino acid, which have many different functional groups. The 20 major amino acids, along with hundreds of other minor amino aids, sustain our lives. Proteins can have interactions with other proteins and biomolecules to form more complex structures and have either rigid or flexible structures for different functions.
Amino Acid[edit | edit source]
Optical Activity[edit | edit source]
All proteins or polypeptides are α amino acids. A typical α amino acid consists of a central carbon (which is the alpha carbon in this case) that is attached to an amino group (-NH2), a carboxylic acid (-COOH), a hydrogen atom, and a distinctive R group. The R groups, usually referred to as the side chains, determine the properties of each amino acid and classify amino acids into different categories. A tetrahedral carbon atom with four distinct groups is called chiral. The ability of a molecule to rotate to the polarized plane to the left is called Levorotatory, and all amino acids exhibit the same configuration as L-isomers. Although D-amino acids (D for Dextrorotatory, meaning that they rotate the plane of polarized light to the right instead), exist naturally, they are not found in proteins. Note: Since the central carbon has four distinct attached groups attached, all of amino acids are chiral except for glycine in which R group is another hydrogen atom.
Zwitterion[edit | edit source]
An amino acid is a zwitterion when it is both "acidic and basic" (amphoteric) as a result of the two functional groups. Zwitterion is the ability of an ion to be positively charged (+) and negatively charged (-). In solid state, the carboxylic acid group deprotonates the amine function protonates, forming a zwitterion (dipolar ion). The structure of an amino acid in aqueous solution depends on the environment pH. The major form in neutral solution is the zwitterion. In strong acid (pH < 1), the predominant form is the cationic ammonium carboxylic acid. In strongly basic solutions (pH > 13), the predominant form is the deprotonated 2-aminocarboxylate ion. These forms interconvert by acid-base equilibria. For pH of about 2 to 9, an amino acid is zwitterion, for which the amino group is protonated (-NH3+) and carboxyl group is deprotonated (-COO-).
Amino Acid Subdivisions[edit | edit source]
Twenty different side chains of amino acids have different functional groups, size, form, charge, capacity for hydrogen bond, hydrophobic nature, and chemical reactivity of proteins. The amino acids can be broadly divided into two catageories, hydrophobic and hydrophilic, according to the chemical properties of the R group. In aqueous environment, the hydrophobic amino acids are unable to participate in hydrogen bonding. They associate with one another and reside mostly inside the protein. On the other hand, hydrophilic amino acids tend to interact in the aqueous environment due to polarity. These amino acids are normally found on the exterior surface of proteins.
- Amino Acids Classification
- Non-polar Amino Acids
- Aliphatic : glycine, alanine, valine, isoleucine, leucine
- Aromatic : phenylalanine, tryptophan.
- Cyclic : Proline
- Polar Amino Acids
- Sulfur-Containing : cysteine, methionine
- Hydroxyl-Containing : serine, threonine
- Aromatic : tyrosine
- Acidic Amide : asparagine, glutamine
- Cyclic Imine : histidine (90%)
- Charged Amino Acids (at physiological pH)
- Negative (acidic) : aspartic acid, glutamic acid
- Positive (basic) : histidine (10%), lysine, arginine
List of 20 Amino Acids[edit | edit source]
| Amino Acid | 3-Letter Abbreviation | 1-Letter Abbreviation | Class of Amino Acid (Side Chain) | Hydrophobicity Index (100 being extremely hydrophobic, 0 being neutral, and -55 being hydrophilic) | Structure | pKa of COOH group | pKa of NH3+ group | pKa of R group | Molecular Weight [g/mol] | alpha helix | beta sheet | Reverse turn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycine | Gly | G | Aliphatic, nonpolar | Neutral (0 at pH = 2; 0 at pH = 7) | 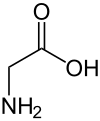
|
2.4 | 9.8 | -- | 75.07 | 0.43 | 0.58 | 1.77 |
| Alanine | Ala | A | Aliphatic, nonpolar | Hydrophobic (47 at pH = 2; 41 at pH = 7) | 
|
2.4 | 9.9 | -- | 89.1 | 1.41 | 0.72 | 0.82 |
| Valine | Val | V | Aliphatic, nonpolar | Very Hydrophobic (79 at pH = 2; 76 at pH = 7) | 
|
2.3 | 9.7 | -- | 117.15 | 0.90 | 1.87 | 0.41 |
| Leucine | Leu | L | Aliphatic, nonpolar | Very Hydrophobic (100 at pH = 2; 97 at pH = 7) | 
|
2.3 | 9.7 | -- | 131.18 | 1.34 | 1.22 | 0.57 |
| Isoleucine | Ile | I | Aliphatic, nonpolar | Very Hydrophobic (100 at pH = 2; 99 at pH = 7) | 
|
2.3 | 9.8 | -- | 131.18 | 1.09 | 1.67 | 0.47 |
| Methionine | Met | M | Hydroxyl or Sulfur-Containing, nonpolar | Very Hydrophobic (74 at pH = 2; 74 at pH = 7) | 
|
2.1 | 9.3 | -- | 149.21 | 1.30 | 1.14 | 0.52 |
| Serine | Ser | S | Hydroxyl or Sulfur-Containing, polar | Neutral (-7 at pH = 2; -5 at pH = 7) | 
|
2.2 | 9.2 | -- | 105.09 | 0.57 | 0.96 | 1.22 |
| Cysteine | Cys | C | Hydroxyl or Sulfur-Containing, polar | Hydrophobic (52 at pH = 2; 49 at pH = 7) | 
|
1.9 | 10.7 | 8.4 | 121.16 | 0.66 | 2.40 | 0.54 |
| Threonine | Thr | T | Hydroxyl or Sulfur-Containing, polar | Neutral (13 at pH = 2; 13 at pH = 7) | 
|
2.1 | 9.1 | -- | 119.12 | 0.76 | 1.17 | 0.96 |
| Proline | Pro | P | Cyclic | Hydrophilic (-46 at pH = 2; -46 at pH = 7) | 
|
2.0 | 9.6 | -- | 115.13 | 0.34 | 0.31 | 1.32 |
| Phenylalanine | Phe | F | Aromatic | Very Hydrophobic (92 at pH = 2; 100 at pH = 7) | 
|
2.2 | 9.3 | -- | 165.19 | 1.16 | 1.33 | 0.59 |
| Tyrosine | Tyr | Y | Aromatic | Hydrophobic (49 at pH = 2; 63 at pH = 7) | 
|
2.2 | 9.2 | 10.5 | 181.19 | 0.74 | 1.45 | 0.76 |
| Tryptophan | Trp | W | Aromatic | Very Hydrophobic (84 at pH = 2; 97 at pH = 7) | 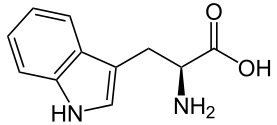
|
2.5 | 9.4 | -- | 204.25 | 1.02 | 1.35 | 0.65 |
| Histidine | His | H | Basic | Hydrophilic at pH=2 (-42), Neutral at pH=7 (8) | 
|
1.8 | 9.3 | 6.0 | 155.16 | 1.05 | 0.80 | 0.81 |
| Lysine | Lys | K | Basic | Hydrophilic (-37 at pH = 2; -23 at pH = 7) | 
|
2.2 | 9.1 | 10.5 | 146.188 | 1.23 | 0.69 | 1.07 |
| Arginine | Arg | R | Basic | Hydrophilic (-26 at pH = 2; -14 at pH = 7) | 
|
1.8 | 9.0 | 12.5 | 174.2 | 1.21 | 0.84 | 0.90 |
| Aspartate | Asp | D | Acidic | Neutral at pH=2 (-18), Hydrophilic at pH=7 (-55) | 
|
2.0 | 9.9 | 3.9 | 133.10 | 0.99 | 0.39 | 1.24 |
| Glutamate | Glu | E | Acidic | Neutral at ph=2 (8), Hydrophilic at pH=7 (-31) | 
|
2.1 | 9.5 | 4.1 | 147.13 | 1.59 | 0.52 | 1.01 |
| Asparagine | Asn | N | Acidic, polar | Hydrophilic (-41 at pH = 2; -28 at pH = 7) | 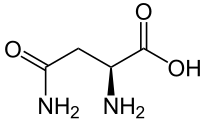
|
2.1 | 8.7 | -- | 132.118 | 0.76 | 0.48 | 1.34 |
| Glutamine | Gln | Q | Acidic, polar | Neutral (-18 at pH = 2; -10 at pH = 7) | 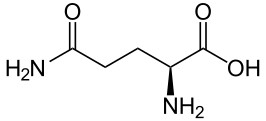
|
2.2 | 9.1 | -- | 146.15 | 1.27 | 0.98 | 0.84 |
Alanine - Ala/ A[edit | edit source]
Structure
Alanine (abbreviated as Ala or A) is an α-amino acid with the chemical formula HO2CCH(NH2)CH3. The α-carbon atom of alanine is bound with a methyl group (-CH3), making it one of the simplest α-amino acids with respect to molecular structure and also resulting in alanine being classified as an aliphatic amino acid. The methyl group of alanine is non-reactive and is thus almost never directly involved in protein function.
Features
Alanine is a nonessential amino acid which meant that it can be manufactured by the human body and does not need to be obtained directly through the diet. Alanine is found in a wide variety of foods, but is particularly concentrated in meats.
Functions
Alanine is the primary amino acids for sugar metabolism. It boosts up the immune system by producing antibodies, and provide energy for muscles.
Chemical Synthesis
Alanine can be manufactured in the body from pyruvate and branched chain amino acids such as valine, leucine, and isoleucine. Alanine is most commonly produced by reductive amination of pyruvate. Because transamination reactions are readily reversible and pyruvate pervasive, alanine can be easily formed and thus has close links to metabolic pathways such as glycolysis, gluconeogenesis, and the citric acid cycle. It also arises together with lactate and generates glucose from protein via the alanine cycle. Racemic alanine can be prepared via the condensation of acetaldehyde with ammonium chloride in the presence of potassium cyanide by the Strecker reaction.
Arginine - Arg/ R[edit | edit source]
Structure
Arginine contained of a four-carbon aliphatic straight chain with the end of which is capped by a guanidinium group. With a pKa of 12.48, the guanidinium group is positively charged in neutral, acidic and even most basic environments. Therefore, arginine has basic chemical properties. Because of the conjugation between the double bond and the nitrogen lone pairs, the positive charge is delocalized and enable the formation of multiple H-bonds.
Features
Arginine is an essential amino acid that plays important role in nitrogen metabolism.
Functions
Arginine assists in wound healing and help in burn treatment. It is necessary in normal immune system activity by enhancing the production of T-cells.
Biosynthesis
Arginine is synthesized from citrulline with the presence of cytosolic enzymes argininosuccinate synthetase and argininosuccinatelyase. This is energetically costly reaction. Therefore, the synthesis of each molecule of argininosuccinate will be coupling with hydrolysis of adenosine triphosphate (ATP) to adenosine monophosphate (AMP).
Synthesis of arginine in human body occurs principally via the intestinal–renal axis, wherein epithelial cells of the small intestine, which produce citrulline primarily from glutamine and glutamate, then join with the proximal tubule cells of the kidney, which extract citrulline from the circulation and convert it to arginine, which comes back to the circulation.
Asparagine - Asn/ N[edit | edit source]
Structure
Asparagine is polar and uncharged derivative of acidic amino acid aspartic acid or aspartate; it has a carboxamide group, which is neutral at physiological pH and can be changed to carboxylic acid by hydrolysis to form aspartate amino acid. The carboxamide group of the amino acid can form hydrogen bonds.
Functions
Asparagine, along with glutamate, is an important neurotransmitter. Since Aspartic acid and Asparigine have high concentration in the hippocampus and hypothalamus of the brain, which is important in short-term memory and emotions, the two amino acids serves essential role between the brain and the rest of the body.
Aspartic acid - Asp/ D[edit | edit source]
Structure
Also known as aspartate, Aspartic acid is an acidic and polar amino acid that has carboxylic acid group, which loses a proton to be carboxylate group for physiological pH and has a negative charge; the carboxylic acid group of the amino acid has a pKa value of 4.1, which is a little basic than the terminal α-carboxyl group.
Features
Aspartic acid is a non-essential amino acid.
Functions
Aspartic acids is involved in transamination in which oxaloacetate and aspartate is interconvertible. It is also involved in immune system activity by promoting immunoglobulin production and antibody production. Moreover, aspartic acid protects the liver and helps in detoxification of ammonia.
Cysteine - Cys/ C[edit | edit source]
Structure
An amino acid that is of sulfhydryl or thiol group, which is more reactive than hydroxyl group, and two of them form stable disulfide bonds. Disulfide bonds linked in cross-way to form polypeptide chain of extracellular proteins by the oxidation of two cysteine residues; the unit of two bonded cysteines is known as cystine.
Functions
Cysteine promotes iron production in iron deficiency anemia. It also assists in lung diseases by increasing production of red blood cells and red blood cells. In addition, Cystein is protective against UV light, radiation, and free radicals production.
Glutamine - Gln/ Q[edit | edit source]
Structure
Glutamine is a polar and uncharged derivative of acidic amino acid glutamic acid or glutamate; it has a carboxamide group, which is neutral at physiological pH and can be changed to carboxylic acid by hydrolysis to form glutamate amino acid. The carboxamide group of the amino acid can form hydrogen bonds.
Functions
Glutamine is a non-essential amino acid meaning it will natually occur in the human body, and it is one of the most abundant amino acid manufacture in the body. Glutamine circulates in the blood and able to cross the blood-brain barrier directly.
Glutamine is critical in gastrointestinal system by providing energy to small intestine function. Interesting, intestine is the only organ in the body that has glutamine as a primary energy source.
Usage
1. Wound Healing 2. Inflammatory Bowel Disease 3. HIV/AIDS 4. Obesity 5. Peritonitis 6. Athletes 7. Cancer 8. etc.
Glutamic acid - Glu/ E[edit | edit source]
Structure
Also known as glutamate, Gluctamic acid is a polar amino acid that has carboxylic acid group, which loses a proton to be carboxylate group for physiological pH and has a negative charge; the carboxylic acid group of the amino acid has a pKa value of 4.3, which is a little basic than the terminal α-carboxyl group and that of aspartic acid.
Function
Glutamic acid is a non-essential amino acid. It plays an important role in DNA synthesis. It also assists in wound and ulcer healing. Glutamic acid take place in the excitatory neurotransmitter and the metabolism of sugars and fats. It aids potassium move through the blood-brain barrier. Glutamic acid are a source of fuel for the brain. Glutamic acid can attach to amine group to form glutamine. The process of forming glutamine will detoxifies ammonia that the body contains.
Glutamic acid is user in correcting personality disorders and treating childhood behavioral disorders. It also take places in treating epilepsy, metal retardation, muscular dystrophy, ulcers, and hypoglycemic coma.
Glycine - Gly/ G[edit | edit source]
Structure
Glycine is the smallest amino acid out of all 20 amino acids For this reason, it has the ability to fit into tight spaces of molecules where no other amino acid could possibly fit therefore glycine is evolutionarily conserved. Most proteins contain small amount of glycine, however collagen is one of the exception that contains 35% glycine. Thus, if glycine were cleaved from an amino acid chain composing a whole protein, it would either alter the function of that protein, or denature it entirely. It is also the only achiral amino acid since its R group is simply a H atom. In particular it does not favor the helix formation.
Functions
Glycione is a non-essential amino acids meaning the human can manufacture it in their body. It serves an important role in maintaining central nervous and digestive systems. Glycine prevents the breakdown of muscle by increase creatine, which is compound that helps build muscle mass. Glycine also keeps the skin firm and flexible. Without glycine, the skin can be damage from the UV rays, oxidation and free radical.
Glycine regulate blood sugar levels and helps provide glucose for the body.
Glycine serve as an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord. When glycine binds to receptors, it activated chloride ion channels to open. As chloride ions enter the channels, the membrane becomes hyperpolarized, causing an Inhibitory postsynaptic potential (IPSP).
Histidine - His/ H[edit | edit source]
Structure
Histidine is a basic, polar amino acid with imidazole group, which is an aromatic ring that can be of positive charge and hydrophilic. The imidazole group of the amino acid has a pKa value of 6, which can be either uncharged or positively charged for about neutral pH. The amino acid is most of time present in active sites of enzymes for the imidazole group can act as acid or base for chemical reactions.
Functions
Histidine is found to be highly concentrated in hemoglobin; thus, it aids in treatment of anemia and maintain optimal blood PH. Also, histidine is the precursor of histamine, which is involved in local immune responses.
Histidine is an essential amino acid meaning the body cannot manufacture by itself. Histadine play important role in stimulating the inflammatory response of skin and mucous membranes. It also stimulates the secretion of the digestive enzymes gastrin and maintain the adequate histamine level.
Isoleucine - Ile/ I[edit | edit source]
Structure
Isoleucine is a nonpolar, aliphatic or hydrophobic amino acid that has two chiral centers for α-carbon atom and the R group; the structure stabilizes water-soluble proteins by hydrophobic effect.
Functions
Isoleucine is an essential amino acid meaning the human body cannot manufacture itself. It is needed for the formation of hemoglobin and regulate blood sugar level and energy level. Isoleucine serves important role in muscle strength and endurance and is a source of energy for muscle tissues.
Leucine - Leu/ L[edit | edit source]
Structure
Leucine has aliphatic R group. It is one of the three amino acids with branched hydrocarbon side chains (generally buried in folded proteins) and result as a nonpolar or hydrophobic amino acid. The hydrophobic effect counts for stabilization of water-soluble proteins.
Features
Leucine is an essential amino acid. It is essential in promoting growth in infant and regulating nitrogen concentration in adults.
Functions
Leucine has all functions of the amino acid Isoleucine as their similarity in branched hydrocarbon side chain. Leucine facilitates skin healing and bone healing by modulating the release of natural pain-reducers, Enkephalins. It is also a precursor of cholesterol and increases the synthesis of muscle tissues by slowing down their degradation process.
Deficiency and Excess
Deficiency of this particular amino acids can result in Hypoinsulinemia, Depression, Chronic fatigue syndrome, Kwashiorkor (or starvation), etc. Excess of Leucine leads to Ketosis.
Lysine - Lys/ K[edit | edit source]
Structure
Lysine has a positively charged amine group chain. The ε-amino group has a significant high pKa value of about 10.8, which is more basic than the terminal α-amino group. This basic amino group is highly reactive and parcipates in the reactions at the active center of enzymes. Although the terminal ε-amino group is charged under physiological condition, the hydrocarbon side chain with three methylene group is still hydrophobic.
Features
Lysine is a naturally occurring essential amino acid in human body. It promotes optimal growth of infants and nitrogen equilibrium in adults.
Functions
Lysine can be a treatment of Herpes Simplex and virus-associated Chronic Fatigue Syndrome as it inhibits viral growth. It facilitates the formation of collagen, which is the main component of fascia, bone, ligament, tendons, cartilage and skin. It also helps in absorption of calcium, which is critical in bone growth of infants.
Deficiency and Excess
Deficiency of lysine is seen in Herpes, Chronic Fatigue Syndrome, AIDS, Anemia, hair loss, and weight loss, etc. Having excessive lysine can result in high concentration of ammonia in the blood.
Methionine - Met/ M[edit | edit source]
Structure
Methionine is one of the two amino acids with side chain containing sulfur. Like Cysteine, the chemical linkage of the sulfur is a thiol ether. Unlike Cysteine, the sulfur do not participate in covenlent bonding to other chemicals. The high inclination of the sulfur oxidation in methionine is one of the causes of smoking-induced emphysema in the human lung tissue.
Features
Methionine is a naturally occurring essential amino acid, which plays critical role in supplying methyl group and sulfur in metabolism. It is also one of only two amino acids coded for by a single codon.
Functions
Methionine helps breakdown of fat and reduce blood cholesterol levels. It is an antioxidant that neutralizes free radicals and removes toxic waste in the liver. Synthesis of DNA and RNA requires the presence of Methionine. It is also precursors of several critical amino acids, hormones, and neurotransmittors in human body. Its AUG codon also serves as a "start" signal for ribosomal translation of messenger RNA; this means that every peptide chain began with an methionine residual at its N-terminal. It may however be removed later on by cleavage.
Deficiency and Excess
Methionine deficiency can be seen in chemical exposure and vegetarians. Severe liver disease can be result of having excessive methionine.
Phenylalanine - Phe/ F[edit | edit source]
Structure
The side chain of Phenylalanine has a derivative of alanine and a phenyl group on the β carbon. Phenylalanine has a stronger hydrophobic characteristics compared to the other amino acids that contain aromatic side chain, tyrosine and tryptophan. Tyrosine and tryptophan are less hydrophobic than phenylalanine due to their hydroxyul and NH side groups. The property of hydrophobicity allows the structure of phenylalanine to be almost always buried in the protein. The electrons of the phenyl ring stabilizes the protein by stacking to other aromactic structures.
Features
Phenylalaine is a naturally occurring amino acid that promote growth in infants and regulate nitrogen concentration in adults.
Functions
Phenylalaine is the precursor of amino acid tyrosine, which give rise to neutrotransmiters, such as dopamine, norepinephrine and epinephrine. It can be used to manage certain types of depression as a powerful anti-depressant and is found to be able to enhance memory, thought, and mood. This amino acid also plays a role in increasing blood pressure in hypertension. All other amino acids are beneficial to human body in L form; the D form of Phenylalanine can be used to reduce pain in arthritis.
Deficiency and Excess
Deficiency of Phenylalanine can be seen in depression, AIDS, obesity, Parkison's Disease, etc. People with the genetic PKU cannot process phenylalanine resulting in a buildup of the amino acid which leads to mental retardation and other permanent damages.
Proline - Pro/ P[edit | edit source]
Structure
Proline is one of the twenty DNA-encoded amino acids. It is unique among the 20 protein-forming amino acids because the α-amino group is secondary rather than primary as other amino acid. The distinctive cyclic structure of proline side chain locks its φ backbone dihedral angle at approximately -75°, giving proline an exceptional conformational rigidity compared to other amino acids. Hence, proline loses less conformational entropy upon folding, which may account for its higher prevalence in the proteins.
Functions
Proline behaves as a structural disruptor in the middle of regular secondary structure elements. However, proline is commonly found as the first residue of an alpha helix and in the edge strands of beta sheets. Proline is most commonly found in turns, which may account for the curious fact that proline is usually solvent-exposed although it has a completely aliphatic side chain. Because proline lacks a hydrogen on the amide group, it cannot act as a hydrogen bond donor, only as a hydrogen bond acceptor.
Serine - Ser/ S[edit | edit source]
Structure
This amino acid's R group is a hydroxyl group attached to a CH2 group. The hydroxyl group is polar giving serine polar/hydrophilic properties. It has a pH of 5.68. pKa = 2.21, 9.15 .Serine is a non-essential amino acid which means it can be synthesized by the human body. For instance, serine can be synthesized by glycine.
Function
Serine is found in phospholipids, active sites of trypsin and chymotrypsin. It can synthesize pyrimidines and proteins, cysteine and tryptophan. It is also involved in fat and fatty acid formation, muscle synthesis.
Threonine - Thr/ T[edit | edit source]
Structure
Threonine is a polar and uncharged amino acid, which is of an aliphatic side chain bonded by a hydroxyl group; the amino acid is constructed as a valine amino acid of a hydroxyl group instead of a methyl group, which is more hydrophilic and reactive. The amino acid is also of two chiral centers for the α-carbon atom and the R group. One of the only 2 amino acids with 2 chiral centers is threonine, the other being isoleucine. This results in the formation of 4 stereoisomers because of 2 chiral centers.
Features
Threonine is an essential amino acd, which means it cannot be synthesized by the human body. Humans must ingest it in the form of threonine containing foods.
Functions
Threonine aids the formation of elastin and collagen. In the immune system, threonine helps in the formation of antibodies. It also promotes growth and function thymus glands and absorption of nutrients. In addition, threonine is the precursor to isoleucine.
Tryptophan - Trp/ W[edit | edit source]
Structure
Tryptophan is an amino acid of aromatic group of an indole group bonded to a methylene group as the side chain, which is of two aromatic rings of nitrogen and hydrogen group and is hydrophilic. One of the side chains is 5 membered while the oher is 6, and 2 carbons are shared by both aromatic rings.
Features
Tryptophan is an essential amino acid meaning it cannot be produced by the human body. It usually present in peptides, enzymes, and structural proteins.
Functions
Trytophan is the precursor for various proteins, serotonin and niacin. It also promotes the formation of peptides and proteins.
Tyrosine - Tyr/ Y[edit | edit source]
Tyrosine
Tyrosine is a polar aromatic amino acid that contains a hydroxyl group attached to an aromatic ring. The hydroxyl group is particularly important because these residues are utilized in the phosphorylation of other proteins. Tyrosine is a non essential amino acid meaning it ca be synthesized in the body. It is synthesized using phenylalanine in the body.
Functions
Tyrosine plays crucial roles in the human body: It helps deal with stress by becoming an adaptanogen, which helps minimize effects of the stress syndrome; in drug detoxification such as for cocaine, coffee and nicotine addictions, it reduces withdrawal symptoms and abuse. It assists in treating Vitiligo, pigmentation of skin, and Phenylketonuria, the condition where phenylalanine is not metabolized. In addition, it is effective for depression treatment.
Tyrosine is also important in the production of epinephrine, norepinephrine, seratonin, dopamine, melanin, and enkephalins, which has pain-relieveing effects in the body. It also affects the function of hormones by regulating thyroid, pituatary and adrenal glands. Moreover, tyrosine disattaches molecules that may be harmful to the cells, therefore is an antioxidant.
Deficiency and Excess
Deficiency of tyrosine is seen in low blood pressure, depression, low body temperature.
Valine - Val/ V[edit | edit source]
Structure
Valine is an amino acid with an aliphatic side chain, therefore a hydrophobic side chain. Valine differs from threonine in a way that the OH group of threonine is replaced by a CH3 group. This is a non polar amino acid. It is essential and therefore cannot be produced by the human body. Being hydrophobic this amino acid is found in the interior of proteins.
Features
In animals, valine must be ingested. In plants, it is created by using pyruvic acid converting it to leucine followed by the reductive amination with glutamate. Valine is found in food: soy flour, fish, cheese, meat and vegetables.
Functions
Valine is essential in muscle growth and development, muscle metabolism, and maintenance of nitrogen balance in the human body. It can be used as energy source in glucose and treatment of damage caused by alcohol in the brain.
Deficiency and Excess
Deficiency of valine affects myelin sheets of nerves. Maple Syrup Urine Disease is caused because Leucine, valine and Isoleucine can't be metabolized.