Structural Biochemistry/Protein function/Insulin
Overview[edit | edit source]
Insulin is a hormone secreted by the pancreas that regulates glucose levels in the blood. Without insulin, cells cannot use the energy from glucose to carry out functions within the body. Insulin was first discovered in 1921 by Frederick Grant Banting and Charles Best from extracted substances from the pancreas of dogs in their laboratory. The material was then used to keep diabetic dogs alive, and then used in 1922 on a 14 year old diabetic boy. The FDA approved insulin in 1939. In 1966 insulin was synthesized by Michael Katsoyannis in his laboratory, which marked the first complete hormone to be successfully synthesized. Synthetic insulin is used as a drug to treat diabetes, and the current forms on the market include insulin from bovine and porcine pancreases, but the most widely used is a form made from recombinant human insulin.

Function[edit | edit source]
Insulin is made in the pancreas by beta cells. After the body takes in food, these beta cells release insulin, which enables cells in the liver, muscles and fat tissues to take up glucose and either store it as glycogen or allow blood to transfer it to organs in the body for use as an energy source. This process stops the use of fat as a source of energy. When glucose levels are elevated in the blood, insulin is produced at higher rates by the pancreas in order to maintain normal sugar concentrations in the blood. Without insulin, the body cannot process glucose effectively and glucose begins to build up in the blood stream instead of being transported to different cells . In contrast with elevated levels of glucose in the blood, when there is a deficit of glucose available to the body, alpha cells in the pancreas release glucagon, a hormone that causes the liver to convert stored glycogen into usable glucose which is then released into the bloodstream.
Some of the effects of the insulin on the metabolism include: 1. Controlling cell intake of substances like glucose in many organs like muscles and adipose tissues. 2. Controlling amino acid uptake, thus increasing DNA replication and protein synthesis 3. Altering the activity of enzymatic cells
Other Cellular effects of insulin include: 1. Increasing synthesis of glycogen. Glycogen is a type of storage for glucose and is stored in the liver. Levels of blood glucose determine whether glucose is stored as glycogen or is excreted. Low levels of glucose cause the liver to excrete glucose, while higher levels of glucose allows glucose to be stored as glycogen. 2. Increasing the synthesis and esterification of fatty acids. This is caused by the insulin causing fat cells to convert blood lipids to triglycerides. Esterification is caused when the insulin causes the adipose tissue to convert fats from fatty acid esters. 3. Increasing the esterification of fatty 4. Decreasing protein breakdown (proteolysis) 5. Reducing lipolysis 6. Increasing uptake of substances like amino acid and potassium 7. Relaxing wall of arteries of muscles, which vasodilation 8. Increasing secretion of HCl into the stomach
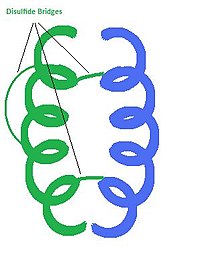
Structure[edit | edit source]
Insulin is a hormone consisting of 2 polypeptide chains. Each chain is composed of a specific sequence of amino acid residues connected by peptide bonds. In humans, chain A has 21 amino acids, and chain B has 30. Post translational modifications result in the connection of these two chains by disulfide bridges. Cysteine residues on A7 and B7, as well as A20 to B19 are covalently connected by disulfide bridges. Chain A also has an internal disulfide bridge connecting A6 to A11. The 3D structure of insulin is composed of 3 helices and the three disulfide bridges. Hydrophobic amino acid residues are clustered on the inside of the molecule while the polar amino acids residues are located on the outer surface. This arrangement of amino acid residues lends stability to the overall molecule. A single molecule of insulin can form a dimer with another insulin molecule, but the most active form is a single unit. The chemical formula for the insulin monomer is: C256H381N65O79S6.

Synthesis[edit | edit source]

Insulin production takes place in the pancreas, however diabetics lack the capability to produce insulin, so insulin derived by synthetic means is required to maintain normal blood glucose levels. Bovine and porcine insulin is similar to human insulin, however insulin synthesized from these sources can have adverse affects when used to treat diabetic patients due to possible long term effects from the continual injection of a foreign substance into the body. As a result of these possible adverse effects, in 1977, researches at the Genentech corporation developed means to reproduce insulin derived from humans through recombinant DNA technology. The steps involved in cloning human insulin begin with extracting proinsulin mRNA from the pancreas of a human with a functioning pancreas. Next, the enzyme reverse transcriptase is used to synthesize a strand of DNA that is complementary to the proinsulin mRNA. This DNA complement is called cDNA. The cDNA and RNA strands form a double helix hybrid. Next, the RNA is hydrolyzed off by raising the pH, and the DNA strand complementary to the original cDNA strand is formed with the help of an enzyme called terminal transferase. Restriction enzymes can be used to cut the gene and isolate just the sequence that encodes for the insulin protein. Next, circular units of DNA, called plasmids, are extracted from E. coli bacteria cells and cut with the same restriction enzyme that was used to cut the human chromosome. Using the same restriction enzymes creates complementary ends on the plasmid and the insulin gene. Next, the insulin gene is inserted into the plasmid at the proper location and the enzyme DNA ligase is used to form the phosphodiester bonds between the insulin gene and the plasmid. This step essentially “glues” the insulin gene into the E. coli plasmid vector. A certain type of plasmid called an “expression vector” is used in this process, which contains a bacterial promoter that facilitates the formation mRNA. Once the insulin genes are ligated into the vector, the vector is inserted into a bacterial cell. The bacterial cell acts as a host for the translation process of mRNA to protein. These host cells are harvested and allowed to reproduce, which creates a colony of insulin producing bacterial cells. The insulin can then be purified and packaged.
Alternative Synthesis[edit | edit source]
Another possible method to synthesize insulin was recently proposed by a pharmaceutical company SynBioSys in 2006 which used safflower to produce insulin rather than bacteria. Their goal ultimately was to reduce economic costs by exceeding its target and achieving accumulation levels of 1.2 percent of total seed protein. The company claims that this breakthrough in plant-produced insulin have the potential to "fundamentally transform the economics and scale of insulin production." The company announces safflower produced insulin can be up to 60% less expensive than insulin manufactured through bacterial cells.
The clinical testing trials were promising: SBS-1000 was bioequivalent to most common brand name insulin medications. SBS-1000 in humans showed no difference in metabolizing bacteria based insulin rather than through safflower. SBS-1000 was well tolerated at pharmacologically active dosages.
Release[edit | edit source]
Insulin is released in the body by the Beta cells in the islets of Langerhans. This is done in two phases, which includes a response in a change in blood glucose level and another type of release which is slower and is independent of sugar.
Insulin released by a change in blood glucose level starts when glucose enters glycolysis and the respiratory cycle. During this cycle, ATP is produced by oxidation, and thus the level of ATP produced is representative of the blood glucose level. When the amount of ATP produced gets to a certain point, potassium channels that are activated by ATP close, depolarizing the cell membrane, leading to a change in other voltage activated channels, such as the calcium channels. Due to the depolarization, voltage gated calcium channels open, allowing an influx of calcium ions into the cell. The increased level of calcium in the cell activates phospholipase C. Phospholipase C cleaves the membrane phospholipid phosphatidyl inositol 4,5-bisphosphate, which in turn becomes inositol 1,4,5-triphosphate and diacylglycerol. The newly formed inositol 1,4,5-triphosphate (also known as IP3) binds to receptors on IP3 gated channels embedded on the membrane of the endoplasmic reticulum. The IP3 gated channels allow an influx of calcium ions in the cell, repolarizing the cell. Insulin, which was synthesized prior to this reaction, is stored in secretory vesicles, and is waiting to be released. The increased levels of calcium due to the binding of IP3 causes the release of the insulin from these vesicles. The beta cells of the islets of Langerhan regulates the glucose level by this reaction. When the blood glucose level is physiologically normal, the beta cells cease to secrete any more insulin. This is done by the sympathetic nervous system, by the release of the hormon norepinephrine.
When blood glucose level drops, hyperglycemic hormones (glucagon) are released by the alpha cells of the Islets of Langerhans. This causes glucose to be released into the blood from storage within the body, and most mainly comes from the liver. The glucose is stored as glycogen within the liver.
Degradation[edit | edit source]
Insulin has two routes of degradation after it has attached to the receptor site on the cell membrane. (1) It may be released into the extracellular environment or (2) it may be degraded by the cell. If insulin is to be degraded by the cell, the insulin-receptor complex is brought into the intracellular area via endocytosis. Subsequently, insulin-degrading enzymes break down the molecule. Insulin is degraded primarily in the liver and the kidneys. The liver is responsible for degrading insulin that is in the bloodstream for the first time; the kidneys are responsible for degrading insulin that is in normal circulation. Natural, endogenously produced insulin is estimated to be degraded within one hour after its initial release into circulation by the pancreatic beta cells. The half life of insulin is approximated to be 4-6 minutes.
Diabetes[edit | edit source]
Diabetes is a condition which the body either cannot produce insulin or does not respond properly to insulin. There are two types of diabetes. Type 1 diabetes is when insulin is not produced by the body. This is due to an autoimmune condition, in which body attacks the Beta cells of the Islets of Langerhans. This can be treated by insulin injections. Type 2 diabetes is when there is a resistance to insulin, reduction in production of insulin, or both.
Insulin is used to maintain a balance of glucose levels in a body’s bloodstream. After a meal, digestion of the carbohydrates occurs and enters the blood as glucose to provide the body with energy. To maintain a body’s blood sugar level, excess sugar is stored in the liver and is released once the blood sugar level starts to become low. This is where diabetes occurs; when the glucose is unable to enter the cells from the pancreas when the sugar levels are too low. If this disease is left untreated, complications, such as blindness and damage to the kidneys can occur.
Non Diabetic Uses of Insulin[edit | edit source]
1) Intravenous feeding solutions: One of the active ingredients in IV solutions used for feeding the body in hospital patients is insulin. The presence of insulin in the boyd helps improve the adsorption of nutrients and when combined with growth hormones, can help reverse negative protein balance.
2) Intravenous GIK solution: Glucose, insulin and potassium solutions have been used to reduce the mortality rate of acute mycoradial infractions, or otherwise known as heart attacks, along with postoperative cardiac failure. By addition of GIK infusions, it is a quick way to infuse potassium into all the cells of the heart even when circulation has ceased completely to readily restore action potential in cardial muscle to induce contraction.
3) Dialysis shock recovery: A bit insulin can be added to an electrolyte solution that can help patients absorb electrolytes quickly to recover from a dialysis shock.
4) Sports Drinks and Oral Rehydration Solutions: The sugars found in these drinks, such as Gatorade, allow for increased secretion of insulin in the body which speeds up absorption of water and electrolytes into all the cells of the body.