Structural Biochemistry/Lipids/Fatty Acids
General Information[edit | edit source]
Fatty acids are key constituent of lipids. Because of the hydrophobic properties that lipids contain, they are able to form membranes within organisms. These lipids possess their Hydrophobicity because of their fatty acids. The overall structure of fatty acids is long hydrocarbon chains of various lengths and degrees of unsaturation terminated with carboxylic acid groups. Some fatty acids have double bonds, which changes the structure. It is said that a fatty acid will usually have an even number of carbons.
Nomenclature[edit | edit source]
The nomenclature of a fatty acid is derived from the name of its parent hydrocarbon by substituting the final e with an oic (i.e., the C18 saturated fatty acid is called octadecanoic acid because its parent hydrocarbon is called octadecane). To number the carbon atoms within a fatty acid, you start at the carboxyl terminus. The C2 and C3 carbon atoms are often referred to as α and β carbons, respectively. At the distal end of the chain, the methyl carbon atom is often referred to as the ω-carbon atom. To denote the position of a double bond in the structure, it is represented by Δ, followed by a superscript number. Alternatively, the double bond can also be presented by counting from the distal end of the chain, with the ω-carbon atom as number 1 in the chain. Fatty acids are referred to according to their carboxylate form because they are ionized at physiological pH.

Saturated Fatty Acid[edit | edit source]

In nature, most fatty acids exist as straight-chain hydrocarbons that attach to a carboxylic acid with the most frequent and even number of carbon atoms. The chain-length range is from 2 to 80 but commonly from 12 up to 24. With a chain length from 2 to 6, they are called short-chain, from 8 to 10 they are called medium-chain, and 12 up to 24 called long-chain fatty acids. Fatty acids are structurally simple and even with their derivatives can be subdivided into well-defined families. Among straight-chain fatty acids, the simplest are referred to as saturated fatty acids. They have no unsaturated linkages in the carbon backbone and cannot be altered during hydrogenation or halogenation process. Saturated fatty acids tend to be solid at room temperature and their melting points increase with increasing chain length.
Saturated fatty acids are most commonly found in animals. The most common saturated fatty acids are Lauric Acid with the chemical composition CH3(CH2)10COOH, Palmitic Acid with the chemical composition CH3(CH2)14COOH, and Stearic Acid with the chemical composition CH3(CH2)16COOH.
Unsaturated Fatty Acid[edit | edit source]
When double bonds are present, fatty acids are said to be unsaturated, monounsaturated if only one double bond is present and polyenoic if they have two or more double bonds generally separated by a single methylene group in the carbon backbone. The configuration for double bond is almost always cis. Therefore, the bent structure is common for unsaturated fatty acids. Unsaturated fatty acids tend to be liquid at room temperature; their melting point increases with increasing chain length but decreases with degree of unsaturation. When a fatty acid is unsaturated and has a short chain length, it increases the fluidity. Most commonly, unsaturated fatty acids are from vegetable origin.
The most common unsaturated fatty acids are Oleic Acid with the chemical composition CH3(CH2)7CH=CH(CH2)7COOH, Linoleic Acid with the chemical composition CH3(CH2)4(CH=CHCH2)2(CH2)6COOH, α-Linolenic Acid with the chemical composition CH3CH2(CH=CHCH2)3(CH2)6COOH, and Arachidonic Acid with the chemical composition CH3(CH2)4(CH=CHCH2)4(CH2)2COOH.

Types of Fatty Acids[edit | edit source]
Monoene acids are fatty acids that contain one double bond. The most common are C-16, C18 and C-22. They also usually have a cis double bond and the double bond typically lies on C-9 for the most important and abundant fatty acids. Oleic acid is one of the most common monoene acids because it is widely distributed and produced. Oleic acid is used as the prototype for all of the monoene acids and also for the n-9 family of polyene acids. Oleic acid can be found in olive oil and several nut oils such as almonds, filberts, cashews, pistachios, pecans and macadamia nuts.
Polyene acids are fatty acids with more than one double bond. Polyene acids that have a methylene-interrupted pattern of unsaturation with 2-6 double bonds and cis configurations are the most important. The two major groups of these methylene-interrupted polyene acids are the n-6 acids based on linoleic acid and the n-3 acids based on alpha-linolenic acid. Linoleic acid is the most common polyene acid because it is used as a prototype for other polyene acids and it is found in most vegetable fats. Alpha-linolenic acid is an essential lipid in leaves, stems and roots.
Another type of fatty acid is the oxygenated fatty acids. The most common oxygenated acid has a hydroxyl, epoxy, or furanoid unit. The most important hydroxyl unit is ricinoleic acid because is the major acid in castor oil, which is used in cosmetics, as a lubricant before and after hydration, and as a drying oil after dehydration. Vernolic acid is the most well-known epoxy acid. It is found in seed oils. Furanoid acids are found at low concentrations in fish oils.
Trans Fats[edit | edit source]
Virtually any candy that we pick from the store, if we care to read the contents says partially hydrogenated oils. The oils used in them are fatty acids. Chemically speaking any long carbon chains with a carboxylic group are fatty acids. By that definition Acetic acid is the smallest fatty acid and naturally occurring fatty acids can be as long as 20 carbons.
Saturated fatty acids are those that have all single bonds except for the keto carbon of the carboxylic group. Unsaturated fatty acids are those that at least have one double bond between the carbons. If the fatty acid has only one double bond, it is referred to as monounsaturated. If it has more than one double bond, it is a polyunsaturated fatty acid.
Cis and trans fatty acids:
All the fatty acids that are found in human body are cis fatty acids, with the exception of retinoic acid (which is present in the eye). If meats or fish are left outside exposed to air, they eventually start to reek. This is partly due to the oxidation of single bonds in the fatty acids, which turns them rancid and is responsible for the bad odor. However if they are saturated fats without single bonds, they do not smell.
Fatty acids with cis double bonds are liquids, although hydrogenation can turn them into solids by turning them into saturated fatty acids. Cis fatty acids have "kinks" in them and hence do not pack well, so they remain in a liquid state at lower temperatures. Saturated fatty acids, however, have straight carbon chains that pack well, which enables them to solidify up to higher temperatures.
The methods of hydrogenation of fats were developed in early 1900s for the purpose of developing solid fats for making soaps. Later they were used to hydrogenate dietary fatty acids such as soybean oils because hydrogenated oils do not go rancid and smell. Slowly, they got into baked goods and candies. And now-a-days, it is difficult to find any packaged foods or snacks without Trans fats.
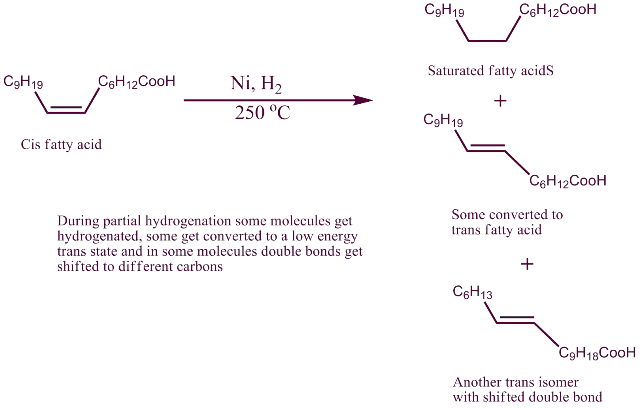
Hydrogenation removes double bonds and not covert cis fatty acids to Trans fatty acid which also contain double bonds.
This leads to the process of hydrogenation:
While there are many modification to it, the major process is to heat the unsaturated oils to above 200 0C, add powdered nickel (as catalyst) bubble hydrogen through it. The double bonds get saturated. However all the double bonds do not get saturated. And at that high temperature some of the double bonds seem to migrate to other carbons in the chain. Formation of trans configuration is more stable than cis. In that process at the newer position they become trans double bond. Cis configuration has more strain in it than trans. As it is seen on the packet ingredient list, they are written as “partially hydrogenated".
Why are partially hydrogenated or trans fats bad for health?
As mentioned above, our body mostly contains cis fatty acids. Whether our cells make them or they are from natural diet sources, they are all of cis configuration. Since all the natural fats are cis, the cellular enzymes have active sites that preferentially metabolize cis fatty acids. So over several years, trans fats accumulate in the body over those of cis form. Since all the natural fatty acids are cis, the enzymes that synthesize triglycerides and the enzymes that breakdown fats for energy, may not work efficiently. If they are not natural molecules, the cell’s enzymes can’t either break them or break them inefficiently. In addition as the trans fats accumulate in the body, as they are similar in structure to cis fats (to an extent) they mat act as competitive inhibitors to fatty acid metabolizing enzymes.
In addition, when natural cis fatty acids are incorporated into the cell membranes, as they have cis configuration, they do not pack very compact thus giving fluidity to the cell membrane. If membranes contain trans fats in them, the membrane fluidity will be affected. It is also likely that membrane receptor function will also be affected.
If the Trans fatty acids are incorporated into erythrocyte membranes, the membranes would be more rigid and erythrocytes would break as they travel through the microcapillaries.
Conclusions based on clinical studies[edit | edit source]
Women with high levels of trans fat in their bloodstreams had three times the risk of developing heart disease as women with the lowest levels of these kinds of fats. C-reactive protein (CRP) is made by the liver. Its levels in the blood are indicators of inflammation. A study of 700 nurses showed that those in the highest quartile of trans fat consumption had blood levels of CRP levels 73% higher than those in the lowest quartile. A 6 year study, monkeys fed with trans fats gained 7.2% body weight compared to 1.8% weight gain in monkeys fed with monounsaturated fats.
Biological Presence[edit | edit source]
Fatty acids in biological systems usually contain an even number of carbon atoms, typically between 14 and 24, although the 16- and 18-carbon fatty acids are the most abundant. Fatty acids typically contain an even number of carbon atoms because of the way in which fatty acids are biosynthesized. Animal fatty acids have hydrocarbon chains which are almost invariably un-branched. The alkyl chain may be saturated or it may contain one or more double bonds. In most unsaturated fatty acids, the double bonds are in the cis formation. The double bonds in polyunsaturated fatty acids, though, are generally separated by at least one methylene group. The chain length and degree of saturation give way to the properties that are found within the fatty acids and lipids. Unsaturated fatty acids have lower melting points than saturated fatty acids of the same length. Because double bonds cause the hydrocarbon chain to bend. Therefore, the fatty acids cannot compact tightly together, reducing the van der Waals interaction between the fatty acids. The melting point of fatty acids is also affected by chain length. The longer the hydrocarbon chain is, the higher the melting point. Short chain length and unsaturation enhance the fluidity of fatty acids and of their derivatives. Animals take advantage of this fatty acid property to maintain the fluidity of their cell membranes. When the weather turns cold, animals have an enzyme that converts saturated fatty acid to unsaturated fatty acid with one or more unsaturation. This prevents the membrane from getting frozen by lowering the melting point of the fatty acids in the membrane. Fatty acids can also form structures known as micelles in an aqueous solution. The structure is formed when the hydrocarbon tails form a hydrophobic center, while the polar heads form a hydrophilic shell outside the interior. The significance of micelles is that they act as emulsifiers, thus dissolving fat-soluble vitamins or other lipids that need to be absorbed.
Essential Fatty Acids[edit | edit source]
There are two fatty acids that the body cannot produce; all of the others can be derived from other molecules. Those two essential fatty acids are linoleic acid and alpha-linolenic acid. Luckily, these two can be found easily in most plant and animal oils. Other fatty acids such as omega-3 fatty acids can be produced by the body, but it is easier to get these from diet. Some sources are fish oils.
These fatty acids are used to help with essential body functions such as blood clotting, immune response, or blood pressure. They help make important fatty acids such as eicosanoids. Eicosanoids are important signaling molecules in the body. They are derived from 20-C chains derived from such molecules as the previously stated omega-3 fatty acids. Eicosanoids participate in activities such as relaying messages in the central nervous system or helping in the inflammatory response.
Other important Fatty Acids[edit | edit source]
Nutritional Significance[edit | edit source]
Fatty acids are significant in the nutrition of living organisms, because of the cell membrane's integral structure built up of fatty acids. Fats can be found in different quantities of various foods. While obesity is becoming a large issue in society today, the reviewing of types of fats and how they affect our bodies is a growing concern. Trans fatty acids are a large part of this concern. Trans fatty acids are fats found in foods such as some cookies, processed foods, crackers, candy, baked goods, fried foods, and other similar items. They are a concern to our health because studies have shown that diets high in trans fats increase the risk of various diseases including heart disease. The trans fats can be related to the levels of LDL cholesterol. Trans fats are found in ingredients labeled as shortening, and hydrogenated oil.
While fatty acids are an important part of living, they can be classified as "good" and "bad" fats. Bad fats are those that have negative effects on cholesterol levels. Bad fats are trans and saturated fatty acids. Good fats are those that have positive effects on cholesterol levels. Good fats consist of poly and monounsaturated fatty acids. Foods such as olive oil, soybean oil, and other vegetable derived oils are usually included. For example Butter consists of 29% Palmitic acid, 9% Stearic acid, 27% Oleic acid, 4% Linoleic acid, and 31% other. While olive oil consists of 6% Palmitic, 4% Stearic, 83% Oleic, and 7% linoleic fatty acids. The difference in the percents of saturated fatty acids between butter and olive oil is significant; butter has an overwhelming amount of saturated fatty acids compared to olive oil's composition being 90% unsaturated fatty acids. Beef as well is largely made up of saturated fatty acids, with 32% palmitic, 25% stearic, 38% oleic, 3% linoleic, and 2% other.
Isolation and Identification[edit | edit source]
The structure of a known acid can be defined by using gas chromatography, comparing the acid with an authentic sample or with compounds of related structure. However, if the fatty acid is completely unknown, spectroscopic procedures will be needed in order to provide more evidence. In order to determine the structure of a fatty acid you must know the chain length and the components of the structure such as branched or cyclic or other functional groups. You must also know the configuration, position, number and nature of the unsaturated centers and also the nature and position of the functional groups.
Thin-Layer Chromatography (TLC)[edit | edit source]
Used more for a qualitative comparison, TLC separates compounds with different polarities based off their attraction to the solvent (mobile phase)which moves up the TLC plate (stationary phase). The compounds have the choice to either react with either phase, which usually have different polar properties. The stationary phase is usually polar silica gel which ties up any of the more polar molecules, essentially slowing their movement up the plate, while the mobile phase is usually of lower polarity to move the less polar compounds father up the plate. Although thin-layer chromatography doesn't separate acids that differ only in chain length or degree of unsaturation, it is still useful in separating acids or esters with additional polar groups. If the silica layer is altered, then other separations can be achieved. One example of this is silver ion chromatography by inserting 5-20% of silver nitrate into the silica. This separates the acids based on the number of double bonds that they have.
High Pressure Liquid Chromatography (HPLC)[edit | edit source]
HPLC systems are essentially based off of the same principles of thin-layer chromatography, but have a more efficient separation process. By using a column with a thin internal diameter, coupled with a high pressured eluent to force the sample through the column, HPLC obtains a higher degree of separation than gravity powered chromatography. There are usually three main types of separation columns employed which are Gel Filtration (Size Exclusion), Ion-Exchange,and Affinity.
Gas Chromatography[edit | edit source]
Gas chromatography using capillary columns are now one of the most commonly used methods in separating methyl esters. By studying the elution out of the capillary column, chain length, degree of unsaturation, and the position of unsaturated centers can be identified.
Biosynthesis of Fatty Acids[edit | edit source]
Understanding the biosynthesis of fatty acids will give you information about the chemical pathway of the fatty acid, the enzymes that are involved in each step of the biosynthesis, the regulatory procedures, and where these reactions occur in the plant, animal, or micro-organism. The five major biosynthetic pathways are: the de novo synthesis of saturated acids from acetate, chain elongation, 9-desaturation to produce monoenes, desaturation in plant systems, and desaturation in animal systems.
Biosynthesis of a Butanoyl Group from Acetyl and Malonyl Building Blocks[edit | edit source]
Step 1: An acetyl group is transferred to the �-carbon atom of the malonyl group with evolution of carbon dioxide. Presumably decarboxylation gives an enol, which attacks the acetyl group.
Step 2: The ketone carbonyl of the acetoacetyl group is reduced to an alcohol function. This reduction requires NADPH as a coenzyme. (NADPH is the phosphate ester of NADH and reacts similarly to it.)
Step 3: Dehydration of the �-hydroxy acyl group.
Step 4: Reduction of the double bond of the �,�-unsaturated acyl group. This step requires NADPH as a coenzyme
De Novo Synthesis of Saturated Acids[edit | edit source]
All of the carbon atoms in fatty acids come from the two carbon atoms in acetate. One is half derived from the original methyl carbon while the other half is derived from the carboxyl carbon atom. Because fatty acids tend to be derived from C2 atoms explain why fatty acids almost always contain an even number of carbons. Malonate is more reactive than acetate and it is produced from acetate and carbon dioxide. Despite the involvement of malonate, it does not appear in the final product and all of the carbon atoms in the fatty acids come from acetate. This acetate-malonate pathway leads to three natural products, which depend on which synthetic pathway is followed. Acetate and malonate produce fatty acids from a reductive pathway, and also phenolic compounds by cyclization of polyacetate and isoprenoids by mevalonic acid. In the de novo pathway, acetate and malonate react in a condensation and reduction cycle, which produces the first C4 acid. The cycle is repeated and each time two more carbons are added until the fatty acid separates from the enzyme system using a hydrolase.
Chain Elongation[edit | edit source]
Chain elongation is very similar to the de novo synthetic route. Chain elongation is different in that the substrate is a preformed fatty acid that is saturated or unsaturated. The substrate reacts with acetyl, condenses, reduces, dehydrates, and reduces again. This produces another acid with another two carbon atoms, which are added at the carboxyl end of the molecule. This is the method by which many fatty acids are converted to long-chain acids such as palmitic acid to stearic acid.
Desaturation to monoene acids[edit | edit source]
Typically, unsaturated acids are produced by aerobic pathways. This occurs by inserting a double bond into a saturated acyl chain in the 9 position. To do this, you remove the pro-R hydrogen (stereospecifically and regiospecifically) from the C9 and C10 carbons to produce a cis alkene.
Desaturation to polyene acids[edit | edit source]
To desaturate further, you must insert more double bonds. In plants, an additional double bond is introduced between the existing double bond and the methyl group, creating a cis configuration. Plants can also introduce double bonds between the existing double bond and the carboxyl group; however this is much less common. Animals are not able to introduce double bonds on the methyl side of the n-9 double bond. Therefore, animals must derive the required linoleate and alpha-linolenate from plant-based dietary sources. Once these are derived, the fatty acids in animals can be desaturated and chain elongated.
Chemical Synthesis of Fatty Acids[edit | edit source]
A lot of the common fatty acids can be collected from natural sources and purchased from chemical suppliers. However, chemical synthesis of fatty acids may necessary if the acids are not easily collected from natural sources because there is no easily accessible source, if acids don’t naturally occur, or if acids are needed in their isotopic form. Saturated acids can be easily formed by chain extension of already available starting acids. This can also be applied to monoene and some other unsaturated acids.
Synthesis via acetylenic intermediates[edit | edit source]
Typically, though, the chemical synthesis of unsaturated acids involves the use of acetylenic intermediates, also known as the Wittig reaction. Acetylene can be alkylated once or twice. Also the triple bond in acetylene can be partially reduced to give cis- or trans-olfenic compounds. The reactivity of alkynes can be extended to give polyenes.
Synthesis by the Wittig reaction[edit | edit source]
In the Wittig reaction, an alkyl halide reacts with a base. This produces the ylid which is condensed using an aldehyde. The result can be either cis- or a trans-isomer. These can be distinguished by selecting certain reaction conditions. At low temperatures, high dilution and without the lithium ion, a cis-isomer is the result of the Wittig reaction. The final product can also be purified using a purification process such as silver ion chromatography.
Isotopically labeled acids[edit | edit source]
In order to produce an isotope of an acid, you must modify the above processes or incorporate into small molecules which are then synthesized. Isotopically labeled acids are essential for the study of reaction mechanisms and lipid biosynthesis and metabolism. The radioactivity of the products that come from the isotopically labeled acids can be analyzed. This is done using mass spectrometry or NMR spectrometry.
Utilization of Fatty Acids as Fuel[edit | edit source]
Peripheral tissues gain access to the lipid energy reserves stored in adipose tissue through three stages of processing. First, the lipids must be mobilized. In this process, triacylglycerols are degraded to fatty acids and glycerol, which are released from the adipose tissue and transported to the energy-requiring tissues. Second, at these tissues, the fatty acids must be activated and transported into mitochondria for degradation. Third, the fatty acids are broken down in a step-by-step fashion into acetyl CoA, which is then processed in the citric acid cycle.
Synthesis and Degradation[edit | edit source]
Although fatty acid synthesis is the reversal of the degradative pathway in regard to basic chemical reactions, the synthetic and degradative pathways are different mechanistically, showing that synthetic and degradative pathways are almost always distinct.
Important differences between the pathways[edit | edit source]
1. Synthesis takes place in the cytoplasm 2. Intermediates in fatty acid synthesis 3. The enzymes of fatty acid synthesis 4. The growing fatty acid chain is elongated 5. The reductant in fatty acid synthesis is NADPH 6. Elongation by the fatty acid synthase complex
References[edit | edit source]
Berg, Jeremy, Biochemistry, 6th Edition
Gunstone, Frank. Fatty Acid and Lipid Chemistry. Glasgow: Blackie Academic & Professional, 1996. 1-34.
http://www.elmhurst.edu/~chm/vchembook/551fattyacids.html
http://www.fda.gov/oc/initiatives/transfat/q_a.html
Chapters 12, 22, Biochemistry, Burg, Tymoczko and Stryer, 6th edition, W. H. Freeman and company
Chapter 19, Organic Chemistry, Vollhardt and Schore, 5th edition, W. H. Freeman and company
http://en.wikibooks.org/wiki/Cell_Biology/Membranes/Hydrophobicity
http://en.wikibooks.org/wiki/Structural_Biochemistry/pH
http://en.wikibooks.org/wiki/Analytical_Forensic_Pharmacology/Melting_Point
http://en.wikibooks.org/wiki/A-level_Chemistry/OCR_(Salters)/Isomerism
http://en.wikibooks.org/wiki/Organic_Chemistry/Introduction_to_reactions/Hydrogenation
http://en.wikibooks.org/wiki/Organic_Chemistry/Chirality/Configurations
http://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme
http://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme/Active_Site
http://en.wikibooks.org/wiki/Structural_Biochemistry/Enzyme/Competitive_Inhibitor
http://en.wikibooks.org/wiki/Structural_Biochemistry/Chemical_Bonding/Van_der_Waals_interaction
http://en.wikibooks.org/wiki/Structural_Biochemistry/Lipids/Micelles
http://en.wikibooks.org/wiki/Structural_Biochemistry/Lipids/Fatty_Acids/Eicosanoids
http://en.wikibooks.org/wiki/Human_Physiology/The_Nervous_System
http://en.wikibooks.org/wiki/Structural_Biochemistry/Lipids/Cholesterol
http://en.wikibooks.org/wiki/Structural_Biochemistry/Chromatography/Gas