Physics Course/Matter
Matter
Physics is the study of phenomena perceived by humans. This perception is due to entities in the nature that have the property of mass and are called as physical objects or matter.
| “ | Matter is any physical entity that has mass | ” |
Such an entity can have other physical properties like shape, size and color and chemical properties. The study of these physical properties and their manifestations is called as Physics.
Characteristics
Physical Properties
The physical properties of matter are the manifestation when the matter is present in bulk. These include mass, volume, density, color, the physical states of the matter, state of motion.
- The mass of an object is the intrinsic property that resists the change of motion of the object; i.e., it is difficult to move a heavier (more massive) object than a lighter object and similarly, it is more difficult to stop a heavy object in motion than a lighter object in motion. This is measured gram (CGS), kilo-gram (SI), mole (SI).
- The volume of an object is the measurement of the amount of the bulk of its constituting matter. It can also be understood as the amount of space occupied by the matter constituting the object. Since gases fill up the entire space they can access. Hence, they do not have a specific volume but have the volume of the container they are in. Volume is measured in cubic units of length.
Chemical Properties
All matter is formed of atoms of one or more types. For example water is a compound of the elements hydrogen and oxygen.
Electrical Properties
Atoms are formed of subatomic particles that are indivisible by any reaction. There are three kinds of these charged particles. Electrons, that carry a negative charge, protons that carry a positive charge and neutrons that carry no charge. The characteristic of three charged particles are shown below
Charged Particles Mass Charge Notation Electron 9.1094 × 10−31 kg −1.602 × 10−19 C e- Proton 1.6726 ×10−27 kg +1.602 × 10−19 C p+ Neutron 1.6726 ×10−27 kg 0 C p0
Matter's Atomic Model
Since charged particles are the smallest particles of a matter therefore a model of matter from charged particles can be developed. There are two models developed by Rutherford and Borh that can be used as a model of matter.
Rutherford's Model
- All matter is made up of chemical elements; atom is the smallest element of matter that cannot be divided by chemical reaction and still has the properties of matter.
- All atoms have a nucleus made of Protons and Neutrons locate in the center and Electrons' orbits circulates around the Nucleus of the Atom
- The number of Electrons on all orbits must be equal to the number of Protons in the Nucleus. The Atomic Number indicates the number of Electrons in orbits or the number of Protons in Nucleus.
- Only Electron in the outer most orbit can participate in any action .
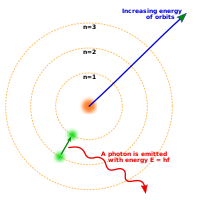
Bohr's Model
- Each orbit of electrons has a quantum energy level correspond to its radius. There are four quantum energy level namely 1,2,3,4 where 1 is the smallest energy level
- The outermost electron orbit has the lowest Quantum Potential Energy E = h f and the greatest Kinetic Energy
- As electron moves from a low potential energy level to a high potential energy level the difference in energy is used to emit Light of Quantum Energy or Photon Light