Adventist Youth Honors Answer Book/Nature/Rocks & Minerals - Advanced
| Rocks & Minerals - Advanced | ||
|---|---|---|
| Nature General Conference |
Skill Level 3 | 
|
| Year of Introduction: 1949 | ||
|
The Rocks & Minerals - Advanced Honor is a component of the Conservation Master Award . |
1. Have the Rocks and Minerals Honor[edit | edit source]
Instructions and tips for earning the Rocks & Minerals honor can be found in the Nature chapter.
2. Have a collection of 30 rocks and minerals properly named, 20 of which you have personally collected. Label with collector's name, date and locality in which it was found.[edit | edit source]
A great time to look for rock and mineral specimens is during a hike. Keep your eyes on the ground, and collect any interesting finds you come across. It is helpful to bring a mason's hammer (for freeing chunks of rock) and a heavy-duty pouch (for storing them). Identification can be accomplished in a number of ways. You could attempt to identify the specimens on your own using a field guide, or you may take the specimens to a local expert who may be able to help you. You can also use a Mineral Identification Key, such as the one found at http://www.minsocam.org/msa/collectors_corner/id/mineral_id_keyi1.htm
3. Know two minerals that belong to each of the following crystal systems[edit | edit source]
a. Isometric[edit | edit source]

The isometric crystal system (or cubic) is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in metallic crystals. Isometric crystals include
- Sodium Chloride (NaCl, common table salt)
- Gallium arsenide (GaAs) is a compound of two elements, Gallium and Arsenic. It is an important semiconductor and is used to make devices such as microwave frequency integrated circuits.
- Zirconium dioxide (ZrO2), sometimes known as zirconia, is a white crystalline oxide of zirconium. Single crystals of the cubic phase of zirconia are commonly used as a substitute for diamond in jewelry. Like diamond, cubic zirconia has a cubic crystal structure and a high index of refraction. Discerning a good quality cubic zirconia gem from a diamond is difficult, and most jewellers will have a thermal conductivity tester to identify cubic zircona by its low thermal conductivity (diamond is a very good thermal conductor).
- Lead(II) nitrate is a chemical compound, the inorganic salt of nitric acid and lead, with the formula Pb(NO3)2. It is a colorless crystal or white powder and a strong, stable oxidizer.
- Copper(I) oxide or cuprous oxide (Cu2O) is an oxide of copper.
b. Hexagonal[edit | edit source]
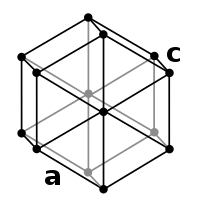
In crystallography, the hexagonal crystal system is one of the 7 lattice point groups (see Hexagonal_lattice). It has the same symmetry as a right prism with a hexagonal base. Hexagonal crystals include:
- Graphite, a form of pure carbon, used as a lubricant and as pencil lead.
- Apatite is one of few minerals that are produced and used by biological micro-environmental systems. Hydroxylapatite is the major component of tooth enamel.
- Quartz is the second most common mineral in the Earth's continental crust. It is made up of a lattice of silica (SiO2) tetrahedra.
- Beryl is a gemstone with the chemical formula Be3Al2(SiO3)6. The hexagonal crystals of beryl may be very small or range to several meters in size.
c. Tetragonal[edit | edit source]
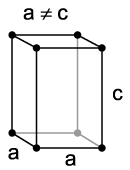
Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (a by a) and height (c, which is different from a). Tetragonal crystals include:
- Wulfenite is a lead molybdate mineral with the formula PbMoO4. It can be most often found as thin tabular crystals with a bright orange-red to yellow-orange color, sometimes brown, although the color can be highly variable. In its yellow form it is sometimes called "yellow lead ore".
- Urea is an organic compound of carbon, nitrogen, oxygen and hydrogen, with the formula (NH2)2CO. It was the first organic compound to be artificially synthesized from inorganic starting materials.
- Zircon is a mineral belonging to the group of nesosilicates. Its chemical name is zirconium silicate and its corresponding chemical formula is ZrSiO4.
4. Know Mohs' scale of hardness and the simplified field tests of hardness. By using these field tests, collect a scale range of specimens from your own region to form your own hardness test set.[edit | edit source]
The Mohs scale of mineral hardness characterizes the scratch resistance of various minerals through the ability of a harder material to scratch a softer material. It was created in 1812 by the German mineralogist Friedrich Mohs and is one of several definitions of hardness in materials science.
Mohs based the scale on ten minerals that are all readily available. As the hardest known naturally occurring substance, diamond is at the top of the scale. The hardness of a material is measured against the scale by finding the hardest material that the given material can scratch, and/or the softest material that can scratch the given material. For example, if some material is scratched by apatite but not by fluorite, its hardness on the Mohs scale is 4.5.
The Mohs scale is a purely ordinal scale. For example, corundum (9) is twice as hard as topaz (8), but diamond (10) almost four times as hard as corundum. The table below shows comparison with absolute hardness measured by a sclerometer.
| Hardness | Mineral | Absolute Hardness |
|---|---|---|
| 1 | Talc
(Mg3Si4O10(OH)2) |
1 |
| 2 | Gypsum (CaSO4·2H2O) | 2 |
| 3 | Calcite (CaCO3) | 9 |
| 4 | Fluorite (CaF2) | 21 |
| 5 | Apatite
(Ca5(PO4)3(OH-,Cl-,F-)) |
48 |
| 6 | Orthoclase Feldspar (KAlSi3O8) | 72 |
| 7 | Quartz (SiO2) | 100 |
| 8 | Topaz (Al2SiO4(OH-,F-)2) | 200 |
| 9 | Corundum (Al2O3) | 400 |
| 10 | Diamond (C) | 1500 |
An improvised hardness scale can be improvised in the field by using things a mineral enthusiast is likely to have on hand. On the Mohs scale, fingernail has hardness 2.5; copper penny, about 3.5; a knife blade, 5.5; window glass, 6.5; steel file, 6.5. Using these ordinary materials of known hardness can be a simple way to approximate the position of a mineral on the scale.
| Hardness | Mineral |
|---|---|
| 2.5 | Fingernail |
| 3.5 | Copper penny |
| 5.5 | Knife blade |
| 6.5 | Window glass or steel file |
5. Do one of the following[edit | edit source]
a. Know and tell two different processes by which metals are extracted from ores.[edit | edit source]
Mineral processing, otherwise known as mineral dressing, is the practice of extracting valuable minerals from their ores.
Industrial mineral treatment processes usually combine a number of unit operations in order to liberate and separate minerals by exploiting the differences in physical properties of the different minerals that make up an ore.
Mineral processing involves four general types of operations: comminution or particle size reduction, sizing or separation of particle sizes by screening or classification, concentration by taking advantage of physical and surface chemical properties, and dewatering or solid/liquid separation.
Comminution[edit | edit source]
Comminution is particle size reduction of materials. Comminution may be carried out on either dry materials or slurries. Crushing and grinding are the two primary comminution processes. Crushing is normally carried out on "run-of-mine" ore, while grinding (normally carried out after crushing) may be conducted on dry or slurried material.
Sizing[edit | edit source]
Sizing is the general term for separation of particles according to size.
The simplest of sizing processes is screening, or passing the particles to be sized through a screen or number of screens. Screening equipment can include grizzlies, bar screens, and wire mesh screens. Screens can be static (typically the case for very coarse material), or they can incorporate mechanisms to shake or vibrate the screen.
Classification refers to sizing operations that exploits the differences in settling velocities exhibited by particles of different size. Classification equipment may include ore sorters, gas cyclones, hydrocyclones, rake classifiers, rotating trommels, or fluidized classifiers. When the feed material contains particles of different densities as well as particles of different size, a degree of concentration takes place during classification because settling velocities are also dependent on particle density.
Froth flotation[edit | edit source]
Froth flotation is achieved when particles are separated based on their surface potential. Hydrophobic particles are recovered to the froth, whereas hydrophilic particles are discharged with the tailings stream. Some mineral particles are naturally hydrophobic, whereas others require specific reagent additions to change their surface potentials. Oxide ores, such as spodumene and tantalite can be treated using oxalic acid based collectors. Sulfide ores can be recovered using xanthate or dithiophosphate type collectors.
Gravity concentration[edit | edit source]
Particles can be classified based on their specific gravity. Gravity concentration processes include:
- Heavy media or dense media separation
- Shaking tables, such as the wilfely table
- Spirals
- Centrifugal bowl concentrators
- Jig concentrators are continuous processing gravity concentration devices.
- Multi gravity separators
- Knelson concentrators
Electrostatic separation[edit | edit source]
Non-conducting particles maintain an electrostatic charge induced electrically, and so remain pinned to a charged drum. Conducting particles do not maintain the electrostatic charge and so fall off the drum, thus minerals such as ilmenite and rutile can be separated.
Magnetic separation[edit | edit source]
Minerals such as magnetite and pyrrhotite are naturally magnetic, and so can be separated from non-magnetic particles using strong magnets.
b. Know eight minerals and tell how each is used.[edit | edit source]
- Diamonds, in addition to their use as a gemstone, have many industrial uses. Because diamond is the hardest mineral known, it is unsurpassed as a cutting implement. They are also used as abrasives.
- Copper is a good conductor of electricity, and as such, it is commonly used in household wiring.
- Silicon can be made to conduct or resist electrical current, and because of this property, it is called a semiconductor. Semiconductors are the foundation of the electronics industry.
- Talc is the first mineral on Moh's scale of hardness. It is ground into a fine dust and used as baby powder.
- Graphite is a form of pure carbon. It is mixed with clay to make pencil leads. It is also used in powdered form as a lubricant.
- Quartz crystals have piezoelectric properties, that is they generate an electric current upon the application of mechanical stress. This electrical current varies at a highly regular rate, making quartz the basis of electrical circuits known as oscillators. Because their oscillation frequency is so stable, quartz crystals are used in clocks and watches.
- Ruby is another gemstone with industrial uses. It is commonly used in lasers.
- Halite is the mineral form of sodium chloride, better known as table salt. It is used not only for flavoring foods, but in lowering the melting temperature of water to cause ice to melt from roadways and sidewalks.
- Fluorite is used instead of glass in some high performance telescopes and camera lens elements. It is also used as a flux to lower the melting point of raw materials in steel production to aid the removal of impurities.
6. Define the following[edit | edit source]
- a. Crystalline
- Having a regular three-dimensional molecular structure
- b. Cryptocrystalline
- Cryptocrystalline is a rock texture which is so finely crystalline, that is, made up of such minute crystals that its crystalline nature is only vaguely revealed even microscopically in thin section by transmitted polarized light. Among the sedimentary rocks, chert and flint are cryptocrystalline. Also a form of diamond, known as carbonado, is cryptocrystalline. Volcanic rocks, especially of the acidic type such as felsites and rhyolites, may have a cryptocrystalline groundmass as distinguished from pure obsidian (acidic) or tachylyte (basic), which are natural rock glasses.
- c. Breccia
- Breccia is a rock composed of angular fragments of rocks or minerals in a matrix, that is a cementing material, that may be similar or different in composition to the fragments. A breccia may have a variety of different origins, as indicated by the named types including sedimentary breccia, tectonic breccia, igneous breccia, impact breccia and hydrothermal breccia.
- d. Noncrystalline
- A noncrystalline material, which has no long-range order, is called an amorphous, vitreous, or glassy material.
- e. Fibrous fracture
- A fibrous fracture occurs when a mineral forms fine, hair-like strands.
- f. Vitreous luster
- A glassy luster or sheen of a mineral surface. The minerals quartz and fluorite exhibit a vitreous luster.
- g. Geode
- A geode is a geological rock formation which occurs in sedimentary and certain volcanic rocks. Geodes are essentially rock cavities with internal crystal formations. The exterior of the most common geodes is generally limestone or a related rock, while the interior contains quartz crystals and/or chalcedony deposits.
- h. Petrification
- Petrification is the process by which organic material is converted into stone or a similar substance. It is approximately synonymous with fossilization. Petrified wood is the most well known result of this process.
- i. Stalactite
- A stalactite is a type of speleothem (secondary mineral) that hangs from the ceiling or wall of limestone caves.
- j. Fluorescence
- Fluorescence is a luminescence that is mostly found as an optical phenomenon in cold bodies, in which the molecular absorption of a photon triggers the emission of another photon with a longer wavelength. For instance, some minerals will glow green when illuminated with an ultra-violet light (green light has a longer wavelength than ultra-violet light).
7. What four metals are frequently found in native or free form?[edit | edit source]
A native metal is any of twenty-one metals that can be found freely in nature. These metals include cadmium, chromium, indium, iron, nickel, tellurium, tin, titanium, and zinc, as well as two groups of metals, the gold group, and the platinum group. The gold group consists of gold, aluminum, copper, lead, mercury, and silver. The platinum group consists of platinum, iridium, osmium, palladium, rhodium, and ruthenium.
8. Discuss the content of three statements from the writings of Ellen G. White concerning rocks or minerals.[edit | edit source]
You can search the writings of Ellen White online at http://egwdatabase.whiteestate.org/nxt/gateway.dll?f=templates$fn=default.htm$vid=default The texts quoted below were found by searching for "diamond," "granite," and "marble." You can discuss these or you might try searching for "jewels," "gems," "gold," "silver," or some other rock or mineral.
- Then I was shown the people upon the earth. Some were surrounded by angels of God, others were in total darkness, surrounded by evil angels. I saw an arm reached down from heaven, holding a golden scepter. On the top of the scepter was a crown studded with diamonds. Every diamond emitted light, bright, clear, and beautiful. Inscribed upon the crown were these words: "All who win me are happy, and shall have everlasting life."
- Below this crown was another scepter, and upon this also was placed a crown, in the center of which were jewels, gold, and silver, reflecting some light. The inscription upon the crown was: "Earthly treasure. Riches is power. All who win me have honor and fame." I saw a vast multitude rushing forward to obtain this crown. - Testimonies for the Church, Volume 1, pp 347,348
- The children of Israel, obedient to the onward movement of the pillar of cloud, left Rephidim, having tarried there some time, and journeyed on toward Sinai. Their line of march had been across open plains, over steep ascents, and through narrow defiles. Again and again, when they had crossed a sandy waste, and their further progress seemed impossible because of the huge piles of massive rocks which lay directly in their way, a narrow passage would appear, and when this was passed, another barren, uninteresting plain would open to their view.
- It was through one of these deep, gravelly passes that they were now called to pass. What a scene was this! Millions of people walled in by abrupt cliffs of granite rocks which rise hundreds of feet on either side, following a moving cloud by day, and guarded at night by a pillar of fire, as if the eye of God were fastened directly upon them. Christ in this wilderness school is here giving his people their first lessons in faith and trust in God. - Signs of the Times, April 22, 1880 par. 1
- The time when David was to be gathered to his fathers had almost come; but before his career closed, he turned his attention to the sanctuary to be erected for the Lord. David was not the one chosen of the Lord to build the temple; but he had no jealousy in his heart on this account, and manifested none the less zeal and earnestness in its behalf. He had prepared in abundance the most costly material,--gold, silver, onyx stones, and stones of divers colors, marble, and the most precious woods. And now all this valuable treasure that he had collected must be committed to others; for other hands must build the house for the ark, the symbol of God's presence. - Review and Herald, December 11, 1888 par. 1
References[edit | edit source]
- Book:Adventist Youth Honors Answer Book/Honors
- Book:Adventist Youth Honors Answer Book
- Book:Adventist Youth Honors Answer Book/Skill Level 3
- Book:Adventist Youth Honors Answer Book/Honors Introduced in 1949
- Book:Adventist Youth Honors Answer Book/Nature
- Book:Adventist Youth Honors Answer Book/General Conference
- Book:Adventist Youth Honors Answer Book/Conservation Master Award
- Book:Adventist Youth Honors Answer Book/Completed Honors