A-level Applied Science/Colour Chemistry/Fibres/Polyamides
Polyamides[edit | edit source]
A polyamide is a polymer containing monomers joined by peptide bonds. They can occur both naturally, examples being proteins, such as wool and silk, and can be made artificially, examples being Nylon, Kevlar and sodium poly(aspartate).
Production from monomers[edit | edit source]
The amide link is produced from the condensation reaction of an amino group and a carboxylic acid or acid chloride group. A small molecule, usually water, ammonia or hydrogen chloride, is eliminated.
The amino group and the carboxylic acid group can be on the same monomer, or the polymer can be constituted of two different bifunctional monomers, one with two amino groups, the other with two carboxylic acid or acid chloride groups.
Amino Acids can be taken as examples of single monomer (if the difference between Alkyl|R groups is ignored) reacting with identical molecules to form a polyamide:

Kevlar (pictured below) is made from two different monomers which continuously alternate to form the polymer:

Nylon[edit | edit source]
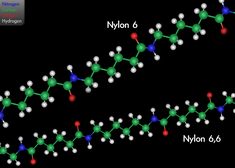 Nylon Nylon
| |
|---|---|
| Density | 1.15 g/cm³ |
| Electrical conductivity (σ) | 10−12 S/m |
| Thermal conductivity | 0.25 W/(m·K) |
| Melting points | 463 K-624 K] 190 °C-350 °C 374 °F-663 °F |
Nylon represents a family of synthetic polymers, a thermoplastic material, first produced on 28 February 1935 by Gerard J. Berchet of Wallace Carothers' research group at DuPont. The first product was a nylon-bristled toothbrush (1938), followed more famously by women's 'nylons' stockings (1940). It is made of repeating units linked by peptide bonds (another name for amide bonds) and is frequently referred to as polyamide (PA). Nylon was the first commercially successful polymer and the first synthetic fibre to be made entirely from coal, water and air. These are formed into monomers of intermediate molecular mass, which are then reacted to form long polymer chains. It was intended to be a synthetic replacement for silk and substituted for it in parachutes after the United States entered World War II in 1941, making stockings hard to find until the war's end. Nylon fibres are now used in fabrics and ropes, and solid nylon is used for mechanical parts and as an engineering material. Engineering grade Nylon is processed by extrusion, casting and injection moulding. Type 6/6 Nylon 101 is the most common commercial grade of Nylon, and Nylon 6 is the most common commercial grade of cast Nylon.
Chemistry of nylon[edit | edit source]
Most nylons are condensation copolymers formed by reacting equal parts of a diamine and a dicarboxylic acid, so that peptide bonds form at both ends of each monomer in a process analogous to polypeptide biopolymers. The numerical suffix specifies the numbers of carbons donated by the monomers; the diamine first and the diacid second. The most common variant is nylon 6,6, also called nylon 66, which refers to the fact that the diamine (hexamethylene diamine) and the diacid (adipic acid) each donate 6 carbons to the polymer chain. As with other regular copolymers like polyesters and polyurethanes, the repeating unit consists of one of each monomer, so that they alternate in the chain. Since each monomer in this copolymer has the same reactive group on both ends, the direction of the amide bond reverses between each monomer, unlike natural polyamide proteins which have overall directionality: C terminal → N terminal. In the laboratory, nylon 6,6 can also be made using adipoyl chloride instead of adipic acid.

It is difficult to get the proportions exactly correct, and deviations can lead to chain termination at molecular weights less than a desirable 10,000 daltons (amu). To overcome this problem, a crystalline, solid "nylon salt" can be formed at room temperature, using an exact 1:1 ratio of the acid and the base to neutralize each other. Heated to 285 °C, the salt reacts to form nylon polymer. Above 20,000 daltons, it is impossible to spin the chains into yarn, so to combat this, some acetic acid is added to react with a free amine end group during polymer elongation to limit the molecular weight. In practice, and especially for 6,6, the monomers are often combined in a water solution. The water used to make the solution is evaporated under controlled conditions, and the increasing concentration of "salt" is polymerized to the final molecular weight.
DuPont patented[1] nylon 6,6, so in order to compete, other companies (particularly the German BASF) developed the homopolymer nylon 6, or polycaprolactam — not a condensation polymer, but formed by a ring-opening polymerisation (alternatively made by condensation polymerisation of aminocaproic acid).


The properties of nylon 6 are sometimes indistinguishable from those of nylon 6,6—except for melt temperature (N6 is lower) and some fibre properties in products like carpets and textiles. There is also a nylon 9.
Other nylons include other copolymerized dicarboxylic acid/diamines. For example, some aromatic nylons are polymerised with the addition of diacids like terephthalic acid (→ Kevlar) or isophthalic acid (→ Nomex), more commonly associated with polyesters.
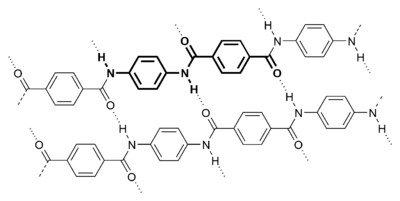
Because of the way polyamides are formed, nylon would seem to be limited to unbranched, straight chains. But "star" branched nylon can be produced by the condensation of dicarboxylic acids with polyamines having three or more amine groups.
The general reaction is:

A molecule of water (or another small molecule e.g. HCl) is given off and the nylon is formed. Its properties are determined by the R and R' groups in the monomers. In nylon 6,6, R' = 6C and R = 4C alkanes, but one also has to include the two carboxyl carbons in the diacid to get the number it donates to the chain. In Kevlar, both R and R' are benzene rings.
Bulk properties of nylon[edit | edit source]
Above their melting temperatures, thermoplastics like nylon are amorphous solids or viscous fluids in which the chains approximate random coils. Below Tm, amorphous regions alternate with regions which are lamellar crystals.[1] The amorphous regions contribute elasticity and the crystalline regions contribute strength and rigidity. The planar amide (-CO-NH-) groups are very polar, so nylon forms multiple hydrogen bonds among adjacent strands. Because the nylon backbone is so regular and symmetrical, especially if all the amide bonds are in the trans configuration, nylons often have high crystallinity and make excellent fibers. The amount of crystallinity depends on the details of formation, as well as on the kind of nylon. Apparently it can never be quenched from a melt as a completely amorphous solid.
Nylon 6,6 can have multiple parallel strands aligned with their neighboring peptide bonds at coordinated separations of exactly 6 and 4 carbons for considerable lengths, so the carbonyl oxygens and amide hydrogens can line up to form interchain hydrogen bonds repeatedly, without interruption. Nylon 5,10 can have coordinated runs of 5 and 8 carbons. Thus parallel (but not antiparallel) strands can participate in extended, unbroken, multi-chain β-pleated sheets, a strong and tough supermolecular structure similar to that found in natural silk and the β-keratins in [eathers. (Proteins have only an amino acid α-carbon separating sequential -CO-NH- groups.) Nylon 6 will form uninterrupted H-bonded sheets with mixed directionalities, but the β-sheet wrinkling is somewhat different. The three-dimensional disposition of each alkane hydrocarbon chain depends on rotations about the 109.47° tetrahedral bonds of singly-bonded carbon atoms.
When extruded into fibres through pores in an industrial spinneret, the individual polymer chains tend to align because of viscous flow. If subjected to cold drawing afterwards, the fibres align further, increasing their crystallinity, and the material acquires additional tensile strength. Block nylon tends to be less crystalline, except near the surfaces due to shearing stresses during formation. Nylon is clear and colourless, or milky, but is easily dyed. Multistranded nylon cord and rope is slippery and tends to unravel. The ends can be melted and fused with a flame to prevent this.
There are carbon fibre/nylon composites with higher density than pure nylon.
Historical uses[edit | edit source]
During World War II, nylon replaced Asian silk in parachutes. It was also used to make tires, tents, ropes, ponchos, and other military supplies. It was even used in the production of a high-grade paper for U.S. currency. At the outset of the war, cotton accounted for more than 80% of all fibres used, and manufactured and wool fibres accounted for the remaining 20%. By August, 1945, manufactured fibres had taken a market share of 25% and cotton had dropped.
Some people, such as Jack Herer, surmise that Cannabis sativa was made illegal because the fibres from the hemp plant, used for fabrics and ropes, were in strong competition with nylon (along with paper, fuel, and other industries). While the production of rope from hemp requires no chemicals or industrial processes, nylon fibre is more than twice as strong as hemp and weighs 25% less. An additional problem is that hemp rope rots from the inside out, making it difficult to determine the condition of a rope at a glance. While hemp was originally used in climbing rope, this is no longer the case, even in countries where cannabis is legal. Even though industrially produced nylon rope is superior than hemp, hemp is a strong alternative to nylons and plastics for many other products, and has the additional advantage of being biodegradable.
Uses of nylon[edit | edit source]
- nylon fibre
- clothing
- tights and stockings
- toothbrush bristles
- fishing lines
- nets
- carpet fibre
- airbag fibre
- auto parts: intake manifolds, gas (petrol) tanks
- slings and rope used in climbing gear
- machine parts, such as gears and bearings
- parachutes
- metallized nylon balloons
- classical and flamenco guitar strings
- paintball marker bolts
- racquetball, squash, and tennis racquet strings
External links[edit | edit source]
- ↑ History of Nylon US Patent 2,130,523 'Linear polyamides suitable for spinning into strong pliable fibers', U.S. Patent 2,130,947 'Diamine dicarboxylic acid salt' issued and U.S. Patent 2,130,948 'Synthetic fibres', all issued 20 September 1938
Silk[edit | edit source]
Silk is a natural protein fiber that can be woven into textiles. It is obtained from the cocoon of silkworm larvae reared in captivity (sericulture). The shimmering appearance for which silk is prized comes from the fibres' triangular prism-like structure, which allows silk cloth to refract incoming light at different angles.
Early history[edit | edit source]
Silk fabric was first developed in ancient China, possibly as early as 6000 BC and definitely by 3000 BC. Legend gives credit to a Chinese Empress Xi Ling-Shi. Though first reserved for the Emperors of China, its use spread gradually through Chinese culture both geographically and socially. From there, silken garments began to reach regions throughout Asia. Silk rapidly became a popular luxury fabric in the many areas accessible to Chinese merchants, because of its texture and lustre. Because of the high demand for the fabric, silk was one of the staples of international trade prior to industrialization.
Animal rights[edit | edit source]
The process of harvesting the silk from the cocoon kills the larvae. Silk has recently come under criticism from some animal rights activists who claims that the common practice of boiling silkworms alive in their cocoons is cruel.
Other uses[edit | edit source]
In addition to clothing manufacture and other handicrafts, silk is also used for items like parachutes, bicycle tires, comforter filling and artillery gunpowder bags. Early bulletproof vests were also made from silk in the era of blackpowder weapons until roughly World War I. Silk undergoes a special manufacturing process to make it adequate for its use in surgery as non-absorbable sutures. Chinese doctors have also used it to make prosthetic arteries. Silk cloth is also used as a material to write on.
External links[edit | edit source]
- Silkworm rearing (with photo journals): [2]
- Scientists have successfully created artificial silk using genetically engineered spider proteins
- What is Dupioni Silk? : [3]
Wool[edit | edit source]
- See Alpaca wool, Angora wool (of rabbits) and Cashmere wool (of goats) for information about other wools.



Wool is the fibre derived from the fur of animals of the Caprinae family, principally sheep and goats, but the hair of certain species of other mammals such as alpacas and rabbits may also be called wool. This article deals explicitly with the wool produced from domestic sheep.
Most of the fibre from domestic sheep has two qualities that distinguish it from hair or fur: it has scales which overlap like shingles on a roof and it is crimped; in some fleeces the wool fibres have more than 20 bends per inch.
The quality of wool is determined by the following factors, fibre fineness, length, scale structure, colour, cleanliness, and freedom from damage. For example merino wool is typically 3-5 inches in length and is very fine. Wool taken from sheep produced for meat are typically more course, larger diameter, and fibres are 1.5 to 6 inches in length. Freedom from damage refers to the structure of the wool when it is removed from the sheep and implies that the wool is clean, white, long, fine, and free of defects from the environment[2].
Both the scaling and the crimp make it possible to spin and felt the fleece. They help the individual fibres attach to each other so that they stay together. Because of the crimp, wool fabrics have a greater bulk than other textiles and retain air, which causes the product to retain heat. Insulation also works both ways; Bedouin and Tuareg use wool clothes to keep the heat out.
The amount of crimp corresponds to the fineness of the wool fibres. A fine wool like merino may have up to a hundred crimps per inch, while the coarser wools like karakul may have as few as one to two crimps per inch.
Hair, by contrast, has little if any scale and no crimp and little ability to bind into yarn. On sheep, the hair part of the fleece is called kemp. The relative amounts of kemp to wool vary from breed to breed, and make some fleeces more desirable for spinning, felting or carding into batts for quilts or other insulating products.
Wool is generally a creamy white colour, although some breeds of sheep produce natural colours such as black, brown (also called moorit) and grey.
Wool straight off a sheep contains a high level of grease (thus "greasy wool") which contains valuable lanolin. In this state it can be worked into yarn or knitted into water-resistant mittens or sweaters, such as those of the Aran Island fishermen. The grease is generally removed for processing by scouring with detergent and alkali. Lanolin removed from wool is widely used in the cosmetics industry.
After shearing, the wool is separated into five main categories: fleece (which makes up the vast bulk), pieces, bellies, crutchings and locks. The latter four are packaged and sold separately. The quality of fleece is determined by a technique known as wool classing, whereby a qualified wool classer tries to group wools of similar gradings together to maximise the return for the farmer or sheep owner.
The fibre diameter of wool varies from 15 micrometres (superfine merino) to 30 or more micrometres for the coarser wools. The finer diameters are generally more valuable.
Due to high concentrations of carbon dioxide, sheep wool does not burn and is therefore also used as an insulation.
Production of wool[edit | edit source]
Global wool production is approximately 1.3 million tonnes per annum of which 60% goes into apparel. Australia, China and New Zealand are leading commercial producers of wool. Most Australian wool comes from the merino breed. Breeds such as Lincoln and Romney produce coarser fibres and wool of these sheep is usually used for making carpets.
In the United States, Texas, New Mexico and Colorado also have large commercial sheep flocks and their mainstay is the Rambouillet (or French Merino). There is also a thriving 'home flock' contingent of small scale farmers who raise small hobby flocks of speciality sheep for the handspinning market. These small scale farmers may raise any type of sheep they wish, so the selection of fleeces is quite wide.
Global wool clip 2004/2005[edit | edit source]
- Australia: 25% of global wool clip (475 million kg greasy, 2004/2005)
- China: 18%
- New Zealand: 11%
- Argentina: 3%
- Turkey: 2%
- Iran: 2%
- United Kingdom: 2%
- India: 2%
- Sudan: 2%
- South Africa: 1%
- United States: 0.77%
(source)
Keeping with the times, organic wool is becoming more and more popular. This blend of wool is very limited in supply and much of it comes from New Zealand and Australia[1].
Uses of wool[edit | edit source]
In addition to clothing, wool has been used for carpeting, felt, insulation (see links) and upholstery. Wool felt covers piano hammers and it is used to absorb odours and noise in heavy machinery and stereo speakers. Ancient Greeks lined their helmets with felt.
References[edit | edit source]
1. Apparel Magazine. Shearing the Edge of Innovation. Jordan K. Speer. May 1, 2006 2.Kadolph, Sara J. Anna L. Langford. Textiles Pearson Education, Inc. Upper Saddle River, NJ 2002
External links[edit | edit source]
Mohair[edit | edit source]
Mohair is a silk-like fabric made from the hair of the Angora goat. Mohair is durable, light and warm. Mohair is used to make sweaters and other clothing and blankets. It is also popular material to make teddy bears.
Mohair should not be confused with the fur from the angora rabbit which is called angora.
The term mohair is often used to describe a type of material used for the folding roof on convertible cars, however in this sense it has no connection with the term's more traditional usage. In these circumstances mohair refers to a form of denim-like canvas.
Angora wool[edit | edit source]
Angora wool is a generic term for either Mohair if the hair is from an Angora goat or Angora fabric if the hair is from an Angora rabbit.
Alpaca wool[edit | edit source]

Alpaca fibre is warmer than sheep's wool and lighter in weight. It is soft and luxurious and lacks the "prickle" factor. However, as with all fleece-producing animals, quality varies from animal to animal, and some alpaca produce fibre which is less than ideal. Fibre and conformation are the two most important factors in determining an alpaca's value.
In physical structure, alpaca fibre is somewhat akin to hair, being very glossy, but its softness and fineness enable the spinner to produce satisfactory yarn with comparative ease. Alpaca fiber can even be spun into yarn with one's fingers.
Alpaca fiber industry[edit | edit source]
In recent years, interest in alpaca fibre clothing has resurged, perhaps partly because alpaca ranching has a reasonably low impact on the environment. Outdoor sports enthusiasts recognize that its lighter weight and better warmth provides them more comfort in colder weather, so outfitters such as R.E.I. and others are beginning to stock more alpaca products. Occasionally, alpaca fibre is woven together with merino wool to attain even more softness and durability. Alpaca fibre is difficult to felt alone but by combining it with merino this is overcome.
The preparing, combing, spinning, weaving and finishing process of alpaca and mohair are similar to that of wool.
Notes[edit | edit source]
References[edit | edit source]
- Hoffman, Dr Eric, The Complete Alpaca Book, published by Bonny Doon Press, 2003
Cashmere wool[edit | edit source]
Cashmere wool is wool obtained from the Kashmir goat. The name derives from an archaic spelling of Kashmir. It is sometimes incorrectly applied to any extremely soft wool, similar to Champagne being used to describe any sparkling wine.
Classification[edit | edit source]
Cashmere is characterized by its soft fibers. For a natural sheep fiber to be considered cashmere, it must be under 19 micrometers in diameter and at least 3.175 centimeters long. It is noted as providing a natural light-weight insulation without bulk. Fibers are highly adaptable and it easily constructed into fine or thick yarns, and light to heavy-weight fabrics. Appropriate for all climates, high moisture content allows insulation properties to change with the relative humidity in the air.
Primary uses[edit | edit source]
Cashmere is used in men's and women's clothing. One of the most notable applications of cashmere is the highly regarded cashmere sweater.
General characteristics[edit | edit source]
Cashmere is characterized as luxuriously soft fiber, For a natural sheep fiber to be considered cashmere, it must be under 19 micrometers in diameter and at least 3.175 centimeters long.It is noted as providing a natural light-weight insulation without bulk. Cashmere is extremely warm (in order to serve its original purpose of protecting goats from cold mountain temperatures.) Fibres are highly adaptable and are easily constructed into fine or thick yarns, and light to heavy-weight fabrics. Appropriate for all climates, a high moisture content allows insulation properties to change with the relative humidity in the air.
The finest fibers are gathered from the saddle of the Cashmere goat; most of the cashmere comes off of the sides and back, from the shoulder to the rump. It is a misconception that the finest fibers come from the neck and belly, as these parts collect the most debris. If the goat is shorn, the fiber must be "de-haired" to remove the coarse, unusable guard hair. Sometimes the fine fibers are collected by combing the goat; either method is time consuming and tedious, thus the high cost of cashmere.
Natural colours[edit | edit source]
The original undyed or natural colors of cashmere wool are various shades of grey, brown and white. Source of the fibre Cashmere wool fiber for clothing and other textile articles is obtained from the Cashmere domestic goat. The goat (Capra hircus laniger) is a mammal belonging to the subfamily Caprinae of the family Bovidae. The goats produce a double fleece consisting of the fine, soft undercoat or underdown of hair mingled with a straighter and much coarser outer coating of hair called guard hair. In order for the fine under down to be sold and processed further, it must first be de-haired. De-hairing is a mechanical process that separates the coarse hairs from the fine hair and after de-hairing the resulting "cashmere" is ready to be dyed and converted into yarn, fabrics and garments . The word 'cashmere' is sometimes incorrectly applied to any extremely soft wool.
Source of the fiber[edit | edit source]
The Cashmere (Kashmir) or down goat is the source of the wool that becomes cashmere fibre for clothing and other textile articles.
The goats produce a double fleece consisting of the fine, soft undercoat or underdown of hair commingled with a straighter and much coarser outer coating of hair called guard hair. In order for the fine underwool to be classified and used as cashmere it must be de-haired. De-hairing is a mechanical process that separates the coarse hairs from the fine hair and after de-hairing the resulting "cashmere" is ready to be dyed to colour and converted into yarn, fabrics and garments.
Production[edit | edit source]
China is the largest producer of raw cashmere and current estimates of production put their annual clip at approximately 10,000 metric tons. Mongolia produces somewhat more than 3,000 tons annually with Iran, Afghanistan, Turkey, Pakistan, India and Central Asian Republics producing significant but lesser amounts. In total the annual world clip is estimated to be in excess of 15,000 but less than 20,000 tons. After the natural animal grease, accumulated dirt and coarse hairs have been removed from the fleece creating "pure cashmere" it is estimated the refined quantity is only about 6,500 tons.
Pure cashmere can be dyed and spun into yarns and knit into sweaters, hats, gloves, socks and other apparel items or woven into fabrics then cut and assembled into garments such as outer coats, jackets, pants, scarves, blankets and other highly luxurious and desirable items.
External links[edit | edit source]

Leather[edit | edit source]
Leather is a material created through the tanning of [hides, pelts and skins of animals, primarily cows. Leather is a very important clothing material, and its other uses are legion. Together with wood], leather formed the basis of much ancient technology. Leather with the fur still attached is simply called fur.
There are a number of processes whereby the skin of a dead animal can be formed into a supple, strong material commonly called leather.
Leather—usually vegetable-tanned leather—can be oiled to improve its water resistance. This supplements the natural oils remaining in the leather itself, which can be washed out through repeated exposure to water. Frequent oiling of leather keeps it supple and improves its lifespan dramatically.
Dyeing leather[edit | edit source]

Leather dying usually involves the use of spirit or alcohol based dyes where alcohol quickly gets absorbed into moistened leather, carrying the pigment deep into the surface. "Hi-liters" and "Antiquing" stains can be used to add more definition to patterns. These have pigments that will break away from the higher points of a tooled piece and so pooling in the background areas give nice contrasts. Leaving parts unstained also provides a kind of contrast.
Alternatives to spirit stains might include a number of options. Shoe polish could be used to dye and preserve leather. Oils, neatsfoot or linseed, can be applied to preserve leather but darkens them. For that reason, a wax paste more often than not serves as the final coat.