A-level Applied Science/Colour Chemistry/Colour
The Chemistry of Colour[edit | edit source]
Coloured chemicals absorb electromagnetic waves in the visible part of the spectrum. The absorbed energy
causes changes in the energy of the molecules’ electrons. The electrons change from a ‘ground
state’ to an ‘excited state’.
Most transitions are not caused by visible light. Many absorb ultra-violet
radiation. Chemicals which absorb UV radiation are colourless (unless they fluoresce). The
energy changes when molecules of a coloured compound and of a colourless compound are illustrated below:
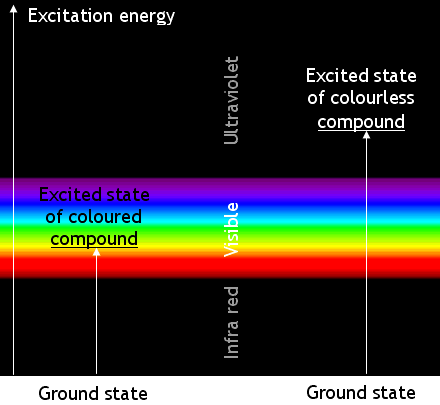
Remember that the apparent colour is caused by absorbing photons of a complementary colour. A
blue compound is blue because it absorbs yellow light.

Chromophores[edit | edit source]
Chemical structures which have excited states corresponding to visible light are called
chromophores. There are two main types:
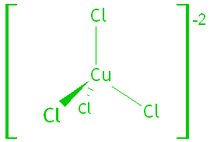
1. Transition Metal Complexes.
Transition metals form complex ions – the metal binds to small molecules
or anions called ligands. The ligands allow the electrons of the metal ion to enter an excited
state if the electrons absorb a photon of visible light.
e.g. tetrachlorocuprate (II) and hexaaquacopper (II) ions:
The partially-occupied d-orbitals of transition metal compounds are important in giving colour to
transition metal complexes. See diagram (which could represent V+2, Cr+3,
Mn+4, etc.):

①. In an uncomplexed ion, all the d-orbitals have the same energy.
②. When ligands surround the ion, the negative charges of the ligands make the d-orbitals
less stable (higher energy).
③. Critically, the ligands will come closer to some d-orbitals than to others. Typically,
two or three of the orbitals will be destabilised more than the remainder.
An electron in one of the lower d-orbitals can acquire the energy to be excited into a higher
d-orbital:

This mechanism allows transition metal complexes to absorb photons of visible light.
2. Conjugated/Delocalised Electron Systems.
When single and double bonds alternate, the electrons in the double bonds can enter an excited
state if they absorb a photon of visible light. e.g. β-carotene (above) has ten conjugated C=C bonds:
The diagram above shows the excitation energies of conjugated aldehydes. n is the number of C=C
double bonds which are conjugated. The simplest (n=1) is CH3-CH=CH-CH=O.
Note how the excitation energy is lower with higher numbers of conjugated bonds.
| n | Wavelength (nm) | Energy (kJ mol−1) |
| 1 | 220 | 544 |
| 2 | 270 | 443 |
| 3 | 312 | 384 |
| 4 | 343 | 349 |
| 5 | 370 | 324 |
| 6 | 393 | 305 |
| 7 | 415 | 289 |
Chromophores of dye molecules often contain unsaturated groups such as >C=O and -N=N-, which are
part of a conjugated bonding system, usually involving aromatic rings. Chrysoidine, a
basic dye, is shown below:

Note how the –N=N- group is just the centre of a conjugated system which extends across all
twelve carbon atoms and includes seven double bonds. All
azo dyes contain the -N=N- arrangement.
Auxochromes: Attached to the chromophore are two -NH2 groups which interact with
the chromophore to modify the orange colour. A group of atoms attached to a chromophore which
modifies the ability of that chromophore to absorb light is called an auxochrome. They can modify
or enhance the colour of the dye. Examples: -OH, - NH2, aldehydes.
Added functional groups can also:
- alter the solubility of the dye in water or other solvents.
- bind the dye molecules to cloth, paper or other substrates.

References[edit | edit source]
Notes on colour chemistry by elecuter.
- ↑ Streitwieser, A & Heathcock, CH (1985) Introduction to organic chemistry (3rd ed) p 628, Macmillan, New York


